Subxiphoid thymectomy: single-port, dual-port, and robot-assisted
Introduction
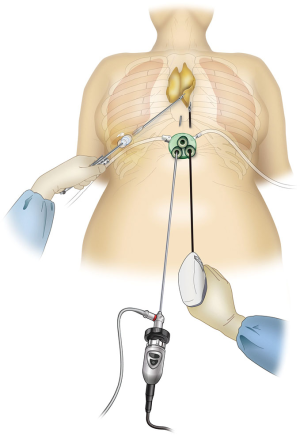
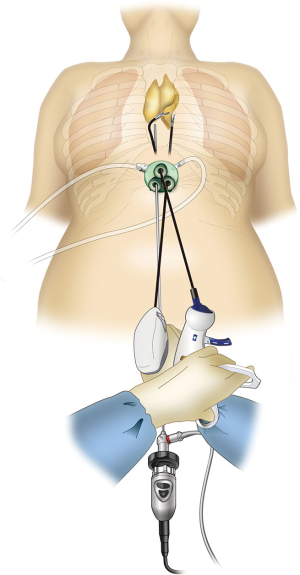
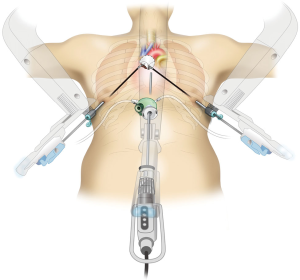
Currently, surgical techniques that are less invasive than conventional median sternotomy are used for thymectomy in the treatment of myasthenia gravis and anterior mediastinal tumors as no sternal incision is required (1). Among the minimally invasive approaches, three are widely used for thymectomy. The first is transcervical thymectomy, reported by Cooper et al. in 1988 (2). This technique has the advantage of causing minimal pain because it does not involve access through the intercostal space. However, surgical operability and field of view are poor, because of which this technique is not widely used. The second is a lateral thoracic approach through the intercostal space, and it is currently the most widely used technique. This approach was introduced in 1992 when Landreneau et al. removed an anterior mediastinal tumor under thoracoscopy (3). It is also commonly used in robot-assisted thymectomy (4,5). The advantage of this technique is that the incision is made in the lateral thoracic region and is inconspicuous. However, drawbacks of this technique include difficulty in intraoperative resection of the cervical thymus and contralateral thymus as well as postoperative intercostal neuropathy (6). The third minimally invasive technique is the subxiphoid approach, which was first reported by Kido et al. in 1999 (7). In 2012, we reported on single-port thymectomy (SPT) using carbon dioxide (CO2) insufflation (8,9). The advantages of this technique are as follows: (I) the field of view offered by the camera scope inserted from the midline of the body helps confirm the location of the superior pole of the thymus and bilateral phrenic nerves; (II) pain is minimal and no intercostal neuropathy occurs because intercostal spaces are not traversed; and (III) cosmetic outcomes are excellent (10). A drawback of this approach is that it requires familiarity with the single-port surgical procedure. Various surgical modifications have been suggested for the subxiphoid approach, which we currently use for thymectomy, including subxiphoid SPT (Figure 1) (8,9); subxiphoid dual-port thymectomy (DPT) wherein an additional lateral thoracic intercostal port is added (Figure 2), which is used for more complicated surgeries (11); and subxiphoid robotic thymectomy (RT; Figure 3) (12,13) using the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA).
Subxiphoid SPT
Patient selection
Thymothymectomy and extended thymectomy are indicated for patients with myasthenia gravis or anterior mediastinal tumors. Because suturing is difficult in single-port surgery, DPT or RT (described below) is indicated in patients who require surgery with suturing, such as pericardial patch closure. If the tumor diameter is <10 cm, the subxiphoid approach is practicable. If the tumor is larger, surgery is performed via a 3-cm incision to prevent the release of pressure from the CO2 insufflation, followed by extension of the skin incision on tumor removal. Combined partial resection of the lung using a stapler can be performed via a subxiphoid single-port approach.
Preoperative preparation
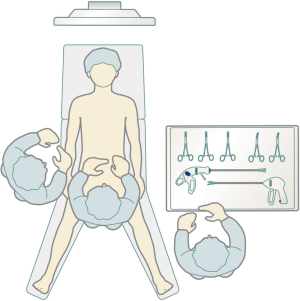
Mechanical ventilation with a single-lumen endotracheal tube is used with the patient under general anesthesia. To prevent the lungs from protruding during surgery and interfering with the procedure, positive-end expiratory pressure is not used. If a tumor has directly invaded the lung and the lung requires combined partial resection, a double-lumen endotracheal tube is used for one-lung ventilation. The peripheral intravenous drip route is secured to the right upper or lower limb, making it possible to clamp the innominate vein in the event of injury to this vein. The patient is placed in an open-legged supine position. The surgeon performs surgery while standing to the patient’s right until the port is inserted, after which surgical procedures are performed with the surgeon standing between the patient’s legs. The scopist operates the camera scope from the patient’s right side. The video monitor is positioned by the patient’s head (Figure 4). In SPT cases, the surgeon must be familiar with operating the 36-cm SILSTM Hand Instruments Clinch (Covidien, Mansfield, MA), which consist of forceps for single-port surgery held in the left hand.
Procedure
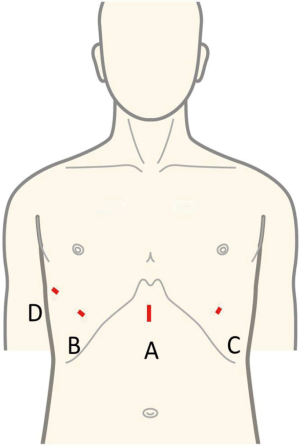
A 3-cm skin incision is made approximately 1 cm caudal to the xiphoid process (Figure 5). Surgery can be performed with either a horizontal or vertical incision. If a horizontal incision is made, the postoperative wound may be less conspicuous as the incision will run along the secant Langer skin line. However, a vertical incision facilitates caudal extension of the skin incision if the tumor is too large to be removed through the initial incision. First, the xiphoid is separated from the rectus abdominis at the site of attachment, after which a finger is inserted behind the sternum to blindly detach the thymus from the sternum. The rectus abdominis fascia can be felt by moving the finger to the caudal end of the incision. Next, an incision is made approximately 1 cm caudal to this location, and space is blindly created to insert a port. The GelPOINT Mini (Applied Medical, Rancho Santa Margarita, CA) is inserted. Three child ports are then inserted in the GelPOINT Mini platform, and CO2 insufflation of the mediastinum is initiated at 8 mmHg. Pressure from the insufflated CO2 causes the pericardium to retract dorsally. The thymus is then separated from the rear surface of the sternum to the cervical region using the LigaSureTM Maryland Jaw 37 cm (Covidien, Mansfield, MA, USA). Generally, we perform all surgical operations with the 36 cm SILSTM Hand Instruments Clinch in the surgeon’s left hand and the LigaSureTM Maryland Jaw 37 cm in the right hand. A 5-mm rigid endoscope camera with a 30° perspective is used. During extended thymectomy, wherein adipose tissue on the left pericardium is difficult to visualize, observation can be facilitated using the EndoCAMeleon (Karl Storz, Tuttingen, Germany), which is capable of a 0°–120° perspective. The bilateral mediastinal pleura are first separated and both sides of the thoracic cavity are opened. Next, the location of the left phrenic nerve is confirmed and the mediastinal pleura, thymus, and surrounding adipose tissue in front of this nerve are detached from the pericardium. In the vicinity of the innominate vein, the thymus is carefully separated from the surface layer while confirming the position of the innominate vein. The thymus anterior to the right phrenic nerve is similarly detached from the pericardium. The positions of the central portion of the innominate vein and right brachiocephalic vein are confirmed in the same manner. The field of view of the neck more cranial to the innominate vein is secured by pulling caudally when the superior pole of the thymus is identified. The thymus in the cervical region is dissected from the thyroid gland on the cranial side, right brachiocephalic vein on the right side, innominate vein on the left side, and brachiocephalic artery and trachea on the dorsal side. Finally, the thymus is pulled either to the left or right to separate the thymic vein and complete the thymectomy. The resected thymus is placed in a bag within the mediastinum and removed through a subxiphoid incision. A 20-Fr drainage tube is then inserted into the mediastinum and surgery is completed (Figure 6).

Subxiphoid DPT
Patient selection
DPT is indicated for patients who require surgery with suturing, including pericardial patch closure, which is difficult with SPT. If a surgeon is unfamiliar with single-port surgery via a subxiphoid approach, DPT is recommended.
Preoperative preparation
Preoperative preparation for DPT is the same as that for SPT. The surgeon generally stands between the patient’s legs and sometimes to the patient’s left or right (Figure 4). The additional port in the lateral thoracic region is a 5-mm port that does not leak CO2.
Procedure
Similar to SPT, the GelPOINT Mini (a port for single-port subxiphoid surgery) is inserted and the thymus is detached from the sternum, after which incisions are made in the mediastinal pleura to open both sides of the thoracic cavity. A 5-mm skin incision is then made on the fifth or sixth intercostal anterior axillary line on either the left or right side (Figure 5). A 5-mm port is inserted while observing the insertion site inside the thoracic cavity using a camera inserted through a subxiphoid port. A left or right intercostal approach is decided depending on the tumor location. The insertion of two ports avoids interference between surgical instruments and allows for more advanced operations, including suturing. Although this technique causes intercostal neuropathy when the port is inserted from the lateral thoracic region through the first intercostal space, confirming the location of the bilateral phrenic nerves is easy and the field of view of the neck is good as a camera view can be obtained from the subxiphoid region at the body midline (Figure 7). Currently, an increasing number of facilities in Japan are using DPT among the different subxiphoid approaches.

Subxiphoid RT
Patient selection
Robotic surgery is problematic considering the high cost. Therefore, performing robotic surgery should have advantages commensurate with the high cost to patients. The existence of multi-articulated forceps in the da Vinci Surgical System facilitates easier surgical operations in narrow spaces. RT is indicated when suturing is needed, which is difficult to perform with SPT.
When invasion of a thymic tumor into the innominate vein is suspected, taping of the vessels of the innominate and right brachiocephalic veins is vital for safety during surgery. A robotic system facilitates this procedure. Median sternotomy is indicated in patients who require vascular sutures, but the easy operability of robot-assisted surgery eventually could make vessel suturing possible.
Preoperative preparation
Surgery is performed with the patient under general anesthesia and in an open-legged supine position. A single-lumen endotracheal tube is used if the tumor has not invaded the lungs. If lung invasion is suspected, a double-lumen endotracheal tube is used for one-lung ventilation. The da Vinci Xi Surgical System would not require docking by the patient’s head, allowing space for the anesthesiologist to manage anesthesia from the patient’s head (Figure 8).
Procedure
Predocking procedure
Similar to SPT described above, an approximately 3-cm skin incision is made 1 cm caudal to the xiphoid process, and the GelPOINT Mini fitted with two child ports is inserted (Figure 5). CO2 insufflation is then performed at 8 mmHg. Using a rigid endoscope with a 5-mm diameter and a 30° perspective, the thymus is separated from the posterior aspect of the sternum with the LigaSureTM Maryland Jaw (Covidien, Mansfield, MA). Incisions are made in the bilateral pleura and both sides of the thoracic cavity are opened. Da Vinci surgical ports are inserted at the anterior axillary line bilaterally through the sixth intercostal spaces while observing the sites of insertion with a thoracoscope inserted through a subxiphoid incision.
If the da Vinci or SI Surgical System is used, it is docked by the patient’s head. The camera is inserted through the subxiphoid GelPOINT Mini. The bilateral intercostal ports are fitted to the da Vinci arms. Generally, I perform surgical operations using bipolar fenestrated grasping forceps in the left hand and bipolar Maryland forceps in the right hand. The EndoWrist Vessel Sealer (Intuitive Surgical), an articulated vessel sealing system, enables use in a more natural direction and allows safe sealing and separation of vessels. The da Vinci system camera scope has no equal magnification and is set at >10× magnification, which can make the field of view appear larger and closer during camera insertion. A surgical assistant assists the surgery through another GelPOINT Mini port. The thymic vein is sectioned using the EndoWrist Vessel Sealer. The resected thymus is placed in a bag within the mediastinum and removed through the subxiphoid incision. A 20-Fr drainage tube is then inserted through the subxiphoid incision, and surgery is completed. The multi-jointed design of the da Vinci system facilitates surgical operations within a narrow mediastinum. Taping of the innominate vein and pericardial patch closure are also easy with this system (Figure 9).

Tips, tricks, and pitfalls
A reason why the subxiphoid approach is not adopted more widely is that respiratory surgeons are unfamiliar with this approach. The trick to inserting a port through the subxiphoid incision is using a finger to create sufficient space behind the sternum and inside the rectus abdominis fascia to insert the port. The pericardium retracts dorsally with CO2 insufflation. Initially, the space behind the sternum is narrow, but as the thymus is separated from the posterior aspect of the sternum, pressure from CO2 insufflation causes this space to expand.
On occasion, the posterior aspect of the sternum is difficult to reach with the LigaSureTM. In such cases, scissors or a similar instrument with a bent tip is used to separate the thymus from the posterior aspect of the sternum. When incisions are made in the bilateral pleura to open both sides of the thoracic cavity, gravity causes the heart to move dorsally, which further expands the space behind the sternum and makes surgery easier. Depending on the patient, it may be difficult to secure the field of view in the cervical region. A trick to securing this field of view is to locate the superior pole of the thymus and pull it caudally to retract the innominate vein. If the field of view is still poor, raising the head end of the operating table will cause the heart to move caudally under the force of gravity and make the cervical region easier to see. In single-port surgery, the camera scope and forceps cross over each other within the port. The route of insertion of the LigaSureTM in the surgeon’s right hand either passes above or below the intersection of the camera scope and gripping forceps. If the tip of the LigaSureTM does not go where intended, it is acceptable to change the route of the LigaSureTM.
DPT is recommended because it has better operability than SPT, which requires operations unique to single-port surgery. DPT only involves one additional 5-mm port in the lateral thoracic region, and it is less invasive than the conventional three-port video-assisted thoracoscopic surgery via a lateral thoracic approach. RT has the best surgical operability among the subxiphoid approaches, but requires the insertion of an additional port in the intercostal space. Surgery becomes easier when an assistant assists the procedure by gripping and pulling the thymus with forceps for single-port surgery. The addition of another port to use a fourth arm also may be acceptable.
The response to bleeding is vital, and countermeasures for bleeding from the innominate vein are important in thymectomy. If bleeding occurs, the first step is to stop the bleeding by applying pressure using the thymus or a swab for thoracoscopy. The GelPOINT Mini child port allows instruments measuring up to 12 mm to be inserted. If bleeding occurs in SPT, the surgeon should switch to DPT if possible, as this will improve operability. An attempt should be made to stop the bleeding by applying a fibrin sheet or other hemostatic agent to the bleeding point. If these do not stop the bleeding, the surgeon should promptly switch to median sternotomy, which is easy as a subxiphoid approach is performed with the patient in a supine position. At some facilities, thymectomy is performed with the patient in the lateral decubitus position using a lateral thoracic approach; however, this position is risky as it is impossible to tape the peripheral end of the innominate vein if bleeding occurs from this vein.
Conclusions
A subxiphoid approach in thymectomy is advantageous to patients as it minimizes or avoids the occurrence of intercostal neuropathy. Moreover, any subxiphoid approach provides surgeons with a good field of view of the cervical region and helps confirm the location of the bilateral phrenic nerves. Therefore, thymectomy using a subxiphoid approach should be considered as a minimally invasive surgical option.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Detterbeck FC, Kim AW, Zielinski M. Looking in from above and up from below: new vistas in thoracic surgery. Innovations (Phila) 2012;7:161-4. [Crossref] [PubMed]
- Cooper JD, Al-Jilaihawa AN, Pearson FG, et al. An improved technique to facilitate transcervical thymectomy for myasthenia gravis. Ann Thorac Surg 1988;45:242-7. [Crossref] [PubMed]
- Landreneau RJ, Dowling RD, Castillo WM, et al. Thoracoscopic resection of an anterior mediastinal tumor. Ann Thorac Surg 1992;54:142-4. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Operative techniques in robotic thoracic surgery for inferior or posterior mediastinal pathology. J Thorac Cardiovasc Surg 2012;143:1138-43. [Crossref] [PubMed]
- Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [Crossref] [PubMed]
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618-25. [Crossref] [PubMed]
- Kido T, Hazama K, Inoue Y, et al. Resection of anterior mediastinal masses through an infrasternal approach. Ann Thorac Surg 1999;67:263-5. [Crossref] [PubMed]
- Suda T, Sugimura H, Tochii D, et al. Single-port Thymectomy through an Infrasternal Approach. Ann Thorac Surg 2012;93:334-6. [Crossref] [PubMed]
- Suda T. Uniportal subxiphoid video-assisted thoracoscopic thymectomy. J Vis Surg 2016;2:123. [Crossref]
- Suda T, Hachimaru A, Tochii D, et al. Video assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results. Eur J Cardiothorac Surg 2016;49:i54-8. [PubMed]
- Suda T, Ashikari S, Tochii D, et al. Dual-port thymectomy using subxiphoid approach. Gen Thorac Cardiovasc Surg 2014;62:570-2. [Crossref] [PubMed]
- Suda T, Tochii D, Tochii S, Takagi Y. Trans-subxiphoid robotic thymectomy. Interact CardioVasc Thorac Surg 2015;20:669-71. [Crossref] [PubMed]
- Suda T. Robotic subxiphoid thymectomy. J Vis Surg 2016;2:118. [Crossref]
- Suda T. Subxiphoid single-port thymectomy in a patient with mature teratoma. Asvide 2017;4:219. Available online: http://www.asvide.com/articles/1529
- Suda T. Using a dual-port system eliminates interference between forceps. Asvide 2017;4:220. Available online: http://www.asvide.com/articles/1530
- Suda T. Subxiphoid robotic thymectomy with pericardial patch closure. Asvide 2017;4:221. Available online: http://www.asvide.com/articles/1531
Cite this article as: Suda T. Subxiphoid thymectomy: single-port, dual-port, and robot-assisted. J Vis Surg 2017;3:75.