Uniportal bilateral video-assisted sequential thoracoscopic extended thymectomy
Introduction
Surgical management of myasthenia gravis (MG) associated with thymic hyperplasia or thymoma is recognized as an effective treatment option. Since the first description of thymectomy in 1941, different approaches have been described, including full median sternotomy, transcervical or partial sternotomy (1-4). Novellino et al. firstly performed multiportal video-thoracoscopic extended thymectomy showing that the complete resection of the thymus and surrounding fat tissue could be achieved even without sternotomy (5). Over the years, several authors demonstrated the feasibility of the procedure having a less post-operative pain, a reduction of hospital stay and an equivalent post-operative mortality and remission rate of MG when compared to sternotomy (6,7). The development of video-assisted thoracic surgery (VATS) technique and surgical devices has permitted several other surgical procedures to be performed through a single-port approach (8-10). Few authors have reported their initial experience using single-port technique for removing thymus or mediastinal masses (6,11-16). Herein we report our initial experience in performing thymectomy in nine patients, using a bilateral sequential uniportal approach without ribs spreading, without any sternum lifting and using traditional laparoscopic instruments.
Patient selection and work up
Eleven consecutive patients underwent uniportal bilateral video-assisted sequential thoracoscopic extended thymectomy for management of MG associated to thymoma or hyperplasia, and thymoma without MG from May 2015 to November 2016. Table 1 shows patients’ characteristics. Of these, two patients were excluded due to an intra-operative diagnosis of thymic carcinoma. All patients underwent the following pre-operative work-up: blood testing, electrocardiography, echocardiography, pulmonary function tests, neurological assessments and radiological evaluation. Chest computed tomography (CT) scan of the neck and chest showed the size, the site and the relationship of the tumor with surrounding tissues. Exclusion criteria from surgery were (I) thymoma bigger than 8 cm involvement; (II) with invasion of surrounding tissue or organs; (III) severe pre-operative co-morbidities that contraindicated resection.
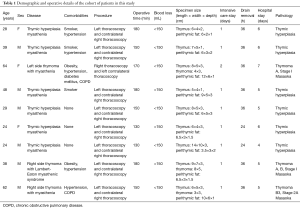
Full table
Pre-operative preparation
Pre-operative preparation for uniportal bilateral video-assisted sequential thoracoscopic extended thymectomy is the same of other uniportal surgical procedure. For patients with MG, pyridostigmine bromide was administered and pre-operative plasmapheresis was performed over a period of 2–5 days as indicated by the neurologist. All patients signed a written informed consent for the operation and were aware that their data could be used anonymously for scientific purpose.
Equipment preference
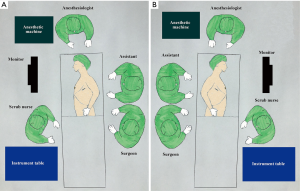
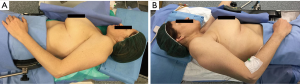
We usually perform a bilateral approach also in patient without MG in order to resect thymoma and all mediastinal fat tissue and reduce the risk of late onset of post-operative MG (17-20). Patients undergoing surgery were sequentially placed in the right and left 60° lateral decubitus position with the ipsilateral arm covered with a sterile stockinet and fixed (almost parallel to the body), to the hip with an adhesive tape, and with a roll placed beneath the shoulder (21). Patients were cleaned and draped as for sterile procedure. Figure 1 shows surgical team placement and patient position during the procedure. The ipsilateral arm was not suspended over the head of the patient to prevent any injury of brachial plexus (Figure 2). The procedure usually starts from the left side and then we change patient position to complete the contralateral dissection. However, in case of thymoma mainly protruding in the left pleural space, we start the procedure from the right pleural cavity and then we completed the resection in the left side. This strategy allows us to reduce the manipulation of the tumor and the risk of capsular rupture and tumor seeding (12). The procedure is performed using a 30° oblique camera, conventional thoracoscopic working instruments and harmonic scalpel (Ultracision Harmonic®, Johnson & Johnson) for tissue dissection and coagulation.
Procedure
Under general anesthesia a left selective endotracheal tube is routinely placed for all thymectomy. An arterial line and a urinary catheter are inserted. Uniportal bilateral video-assisted sequential thoracoscopic extended thymectomy is a totally endoscopic procedure without accessory incision and directly watching the monitor. Surgical dissection is usually started leftward with the patient in the 60° right lateral decubitus. A 3 to 5 cm incision is made at the fourth intercostal space, at the level of the anterior axillary line, without rib spreading. The lung is deflated and through the minithoracotomy the 30° thoracoscope and conventional thoracoscopic instruments are inserted. The camera is placed at the posterior end of the uniportal access. The dissection usually starts from the left pericardiophrenic angle. Proceeding cranially, the next step is to incise the mediastinal pleura along the anterior border of the phrenic nerve and to mobilize the thymus and perithymic fatty tissue. Endoscopic vascular clips are used to control thymic vessels. The dissection is carried out in a pre-pericardial plane. Finally the upper thymic poles are dissected and gently pulled down to obtain their complete mobilization until the thyroid-thymic ligaments became visible to be sectioned. After completion of leftward thymic dissection, we open the contralateral pleura to push the mobilized part of the specimen into the right pleural cavity while lung being in apnea for few seconds. At this point the residual mediastinal fat is fully dissected from the left phrenic nerve, the innominate vein, the aortocaval groove, the aortopulmonary window (APW), and the left pericardiophrenic angle. A single 24 Fr chest tube is inserted through the incision, curved downward toward the costophrenic angle. No rib spreading is used during the entire procedure.
Sequential contralateral side
The patient is then positioned in the contralateral 60°∆ lateral decubitus. Likewise to the left side procedure a 3 to 5 cm incision is made at the fourth intercostal space, at the level of the anterior axillary line, without rib spreading. The dissection starts from the right pericardiophrenic angle and continue cranially along the anterior border of the phrenic nerve. The thymus, or the thymoma, is then mobilized from the surrounding tissue and from superior vena cava. Endoscopic vascular clips are used to control thymic veins arising from superior vena cava. Finally the “en bloc” thymic dissection, with bilateral perithymic and pericardiophrenic fatty tissue is completed and the specimen is removed using a 15 cm endo-bag through the uniportal access. At this point the mediastinal fat is fully dissected from the right phrenic nerve, the innominate vein and the right pericardiophrenic angle. A single 24 Fr chest tube is inserted through the incision curved downward toward the costophrenic angle. The lung is then reinflated. Figures 3-5 show the main steps of three different clinical cases.



Roles of team members
The surgeon and the assistant are positioned behind the patient. The camera is placed at the posterior end of the uniportal access and is handled by the assistant. The camera does not interfere with surgeon instruments and assures a stable view of the operative field. The assistant retreats the camera to avoid lens blurring secondary to coagulation and turn the camera according the need of the surgical step; for example during the dissection of upper thymic poles the camera has to look at toward the thoracic inlet. It is also mandatory that the surgeon action has to be at the center of the screen. Finally the synchronization between the surgeon and the assistant is essential to reduce operative time and to the success of the procedure. The scrub nurse stands on the opposite side of the patient and his/her role is to assist surgeons maintaining the fluency of the operation, to select and pass surgical instruments to the surgeon, to clean the tools after using and paying attention that everything remains sterile. Anesthesiologist stands at the head of the patient monitoring all parameters of the patient. With patients with MG we do not use muscular relaxants, instead we administrate lidocaine spray during intubation (25).
Postoperative management
Our post-operative analgesic strategy is to produce an intercostal block with ropivacaine 1.0% (dose: 10 mg/mL). Intercostal injection is administrated within 2 min at the rate of 4 mL in the fourth intercostal space, 3 mL in the third and fifth intercostal space at closure time. Furthermore, at the end of surgery a 100 mL elastomeric infusion pump (ketorolac 120 mg + morphine chlorhydrate 40 mg + ondansetron 8 mg) with a flow rate of 2.5 mL/h is connected for 48 h. Patient starts oral feeding the day after and is mobilized on post-operative day 1. Patients with MG continued their therapy, in particular pyridostigmine bromide monitored by the anesthesiologist under the guidance of the neurologist. Patients are fully mobilized in post-operative day 1. Chest drain are removed when the drainage volume is <100 mL/die.
Tips, tricks and pitfalls
- After selective intubation, a nelaton catheter on continuous suction is positioned into the double lumen side where the surgeon is working to obtain maximal lung deflation and constantly aspirating sputum. CO2 insufflation was not needed to collapse the lung.
- The surgical incision should be made at the superior border of fifth rib in order to respect intercostal muscle and to enter directly into the pleural cavity avoiding to create little muscular flaps bleeding and redounding on the port access. Furthermore skin incision is a crucial step to determine how easy or complex the procedure would be.
- Thoracoscopic exploration is particularly important in case of thymoma to detect the presence of a complete capsule and clear border. In case of macroscopic capsular involvement or invasion of surrounding organs a sternotomy should be performed.
- Anatomical landmarks for thymus dissection are right and left phrenic nerve, the tissue above the innominate vein and superior vena cava.
- During thymic dissection while retracting the thymus with the left hand, the surgeon’s right hand dissect mediastinal tissue with harmonic scalpel (Ultracision Harmonic®, Johnson & Johnson). A yankauer cannula is sometime useful as tool for blunt dissection especially while dissecting sanguinolent tissue (Figures 4,5). Endoscopic “peanut gauze” can also be used (Figure 5). Atraumatic bowel graspers are used to retract the thymus during dissection. Sometimes it is difficult to reach with instruments where the operator intends to go or instruments are not advancing in the intended direction. In this case change tools positioning, i.e., change the camera and place it at the anterior end of the uniportal access or instruments direction, could be useful to overcome the difficulty.
- Dissection of the entire thymic poles is mandatory to obtain a radical surgical procedure and for this step a 30° thoracoscope offers a superior operative vision of the thoracic inlet. During this time the downward compression of paratracheal cervical tissue pushes thymic poles down and facilitates its dissection. Furthermore, bringing the superior thymic poles (especially if long) with an atraumatic bowel grasper and rotating it anti clockwise can be very useful in pulling down them (Figures 3,5).
- Thymic dissection is usually carried out from costophrenic angle toward the thoracic inlet but when the dissection is difficult due to hard tissue or strong tissue adhesion, strategy can change and dissection performed where possible. Do what is easy first!
- Our specific patient’s positioning with the arm fastened to the hip allows the surgeon to change his position and work easily also on the cardiophrenic region putting himself cranially to the head of the patient at the place of the anesthesiologist if necessary. Furthermore this position avoids any possible brachial plexus injury.
- Perivascular fatty tissue dissection is one of the most demanding step of thoracoscopic thymectomy. Particularly, fat surrounding left innominate vein has to be removed with attention to avoid vascular injury. In any case, step by step meticulous hemostasis determine a bloodless field and reduce the risk of vascular injury. In case of minor bleeding from the innominate vein, pressure is applied with a radiopaque 10 cm × 10 cm gauze swab (Figure 4). If hemostasis is not achieved, immediately switch VATS to a lateral thoracotomy or a median sternotomy depending on to the vascular injury position and surgeon experience. In case of massive bleeding is mandatory to have 1–2 long aortic vascular clamps ready on the instrument table. Anyway according to us the most dangerous anatomic pitfalls for bleeding is the junction between internal mammary vein and innominate vein.
- At the end of both left and right uniportal thymectomy, the procedures have to be completed removing the mediastinal fat near the phrenic nerves, the innominate vein, the aortopulmonary window and pericardiophrenic angles.
With uniportal access the surgeon should always maintain the direct vision of active surface of the harmonic scalpel or of scissor to prevent unexpected tissue injury during dissection. Standardization of technique, maintain the same surgical team, could be a very useful trick to obtain a good surgical performance and consequently a good surgical outcome. For surgeon who are unfamiliar with single-port thymectomy, we recommend first commencing with a larger (8–10 cm) uniportal access without rib spreading trying to reduce the size of thoracotomy in the next case. With this method the surgeon learn the secret of different eye-hand coordination compared to multiportal procedure.
Conclusions
Uniportal bilateral video-assisted thoracoscopic extended thymectomy is a safe and feasible technique for surgical resection of thymic hyperplasia and thymoma. This technique could be considered a development of multiportal thoracoscopic thymectomy offering all advantages of minimally invasive surgery, such as less post-operative pain, faster patient mobilization, shorter hospital, an increased satisfaction and even a better cosmetic results. Furthermore we think that the evolution from multiportal to anterolateral uniportal thymectomy may be less difficult than the passage to the subxiphoid uniportal approach as there is no change of surgical view comparing uniportal and multiportal technique.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Blalock A, Harvey AM, Ford FR, et al. The treatment of myasthenia gravis by removal of the thymus gland: preliminary report. JAMA 1941;117:1529-33. [Crossref]
- Kirschner PA, Osserman KE, Kark AE. Studies in myasthenia gravis. Transcervical total thymectomy. JAMA 1969;209:906-10. [Crossref] [PubMed]
- Kark AE, Kirschner PA. Total thymectomy by the transcervical approach. Br J Surg 1971;58:321-6. [Crossref] [PubMed]
- Miller JI, Mansour KA, Hatcher CR Jr. Median sternotomy T incision for thymectomy in myasthenia gravis. Ann Thorac Surg 1982;34:473-4. [Crossref] [PubMed]
- Novellino L, Longoni M, Spinelli L, et al. "Extended" thymectomy, without sternotomy, performed by cervicotomy and thoracoscopic technique in the treatment of myasthenia gravis. Int Surg 1994;79:378-81. [PubMed]
- Scarci M, Pardolesi A, Solli P. Uniportal video-assisted thoracic surgery thymectomy. Ann Cardiothorac Surg 2015;4:567-70. [PubMed]
- Rückert JC, Walter M, Müller JM. Pulmonary function after thoracoscopic thymectomy versus median sternotomy for myasthenia gravis. Ann Thorac Surg 2000;70:1656-61. [Crossref] [PubMed]
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [Crossref] [PubMed]
- Berlanga LA, Gigirey O. Uniportal video-assisted thoracic surgery for primary spontaneous pneumothorax using a single-incision laparoscopic surgery port: a feasible and safe procedure. Surg Endosc 2011;25:2044-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fieira E, et al. Single-incision video-assisted thoracoscopic lobectomy: initial results. J Thorac Cardiovasc Surg 2012;143:745-7. [Crossref] [PubMed]
- Jiang L, Liu J, Shao W, et al. Non-intubated subxiphoid uniportal video-assisted thoracoscopic thymectomy using glasses-free 3D vision. J Thorac Dis 2016;8:E1602-4. [Crossref] [PubMed]
- Caronia FP, Fiorelli A, Arrigo E, et al. Bilateral single-port thoracoscopic extended thymectomy for management of thymoma and myasthenia gravis: case report. J Cardiothorac Surg 2016;11:153. [Crossref] [PubMed]
- Wu CY, Heish MJ, Wu CF. Single port VATS mediastinal tumor resection: Taiwan experience. Ann Cardiothorac Surg 2016;5:107-11. [Crossref] [PubMed]
- Wu L, Lin L, Liu M, et al. Subxiphoid uniportal thoracoscopic extended thymectomy. J Thorac Dis 2015;7:1658-60. [PubMed]
- Caronia FP, Fiorelli A, Santini M, et al. Uniportal bilateral video-assisted thoracoscopic extended thymectomy for myasthenia gravis: A case report. J Thorac Cardiovasc Surg 2015;150:e1-3. [Crossref] [PubMed]
- Wu CF, Gonzalez-Rivas D, Wen CT, et al. Single-port video-assisted thoracoscopic mediastinal tumour resection. Interact Cardiovasc Thorac Surg 2015;21:644-9. [Crossref] [PubMed]
- Rowland LP, Aranow H Jr, Hoefer PF. Myasthenia gravis appearing after the removal of thymoma. Neurology 1957;7:584-8. [Crossref] [PubMed]
- Sun XG, Wang YL, Liu YH, et al. Myasthenia gravis appearing after thymectomy. J Clin Neurosci 2011;18:57-60. [Crossref] [PubMed]
- Kondo K, Monden Y. Myasthenia gravis appearing after thymectomy for thymoma. Eur J Cardiothorac Surg 2005;28:22-5. [Crossref] [PubMed]
- Kang SY, Lee JS, Choi JC, et al. Myasthenia gravis appearing after thymectomy: a case report and review of the literature. J Clin Neurol 2007;3:158-60. [Crossref] [PubMed]
- Caronia F, Fiorelli A, Monte AL. Bilateral thoracoscopic thymectomy using a novel positioning system. Asian Cardiovasc Thorac Ann 2014;22:1135-7. [Crossref] [PubMed]
- Caronia FP, Arrigo E, Trovato S, et al. The entire technique of bilateral uniportal video-assisted sequential thoracoscopic extended thymectomy in a patient with thymoma without myasthenia gravis, with Lambert-Eaton myasthenic syndrome. Asvide 2017;4:200. Available online: http://www.asvide.com/articles/1510
- Caronia FP, Arrigo E, Trovato S, et al. The entire technique of bilateral uniportal video-assisted sequential thoracoscopic extended thymectomy in a patient with myasthenia gravis and thymic hypertrophy. Asvide 2017;4:201. Available online: http://www.asvide.com/articles/1511
- Caronia FP, Arrigo E, Trovato S, et al. The entire technique of bilateral uniportal video-assisted sequential thoracoscopic extended thymectomy in a patient with myasthenia gravis associated to a little thymoma. Asvide 2017;4:202. Available online: http://www.asvide.com/articles/1512
- El-Dawlatly AA. Anesthesia for thoracoscopic thymectomy: modified non-muscle relaxant technique--case reports. Middle East J Anaesthesiol 2007;19:219-24. [PubMed]
Cite this article as: Caronia FP, Arrigo E, Trovato S, Lo Monte AI, Cottone S, Sgalambro F, Guglielmo M, Volpicelli A, Fiorelli A. Uniportal bilateral video-assisted sequential thoracoscopic extended thymectomy. J Vis Surg 2017;3:69.


