Robotic thymectomy
Introduction
Thymectomy is the main operation in the treatment of thymomas. In addition, since in the first decades of the twentieth century was observed an improvement of myasthenia gravis (MG) after thymectomy, this procedure has gained importance in the multidisciplinary treatment of MG, becoming a widely accepted therapeutic option (1-3).
Different surgical approaches have been described for this operation, either open (sternotomy, thoracotomy) or minimal invasive ones (cervicotomy, video-assisted thoracoscopic surgery, VATS). Anyway, median sternotomy still represents the gold standard.
The use of robotic systems in the surgical field represented an important innovation for minimally invasive techniques, enabling to overcome limitations of standard approaches (4).
In 1999, Loulmet and Reichenspurner described the first application of a surgical robotic system, performing a coronary by-pass (5,6). Yoshino performed the first robotic thymectomy for a small thymoma in 2001 (7). Rea and Ashton both described in 2003 the first series of patients undergoing robotic thymectomy for MG, using the latter a right-sided approach with completion of the operation through the left side, while the former used only a left-side approach (8,9). In the following years, different authors described their results with robotic thymectomy both for thymic tumors and in cases of nonthymomatous myasthenia gravis (10-12). Analysis of literature data shows that robotic approach in thymectomy is a feasible and safe operation. In case of patients with MG, this technique shows encouraging long-term results. On the other side, in patients affected by early stage thymoma, the technical and oncological results seem promising, but there is the need of longer follow-up data (10,13).
Patient selection and workup
Main indications for robotic thymectomy are patients with MG and patients with early stage thymic tumors associated or not with MG.
During pre-operative evaluation, it is important to investigate whether there are symptoms or clinical signs that could be related to MG and to evaluate the serum titer of antibodies against acetylcholine-receptor. If negative, anti-MuSK (muscle specific receptor tyrosine kinase) antibodies should be tested; there is evidence that a positive serum titer of anti-MuSK Ab is predictive of a lesser effect of thymectomy on MG symptoms (14).
Anyway, neurological assessment prior to surgery should be always performed to evaluate presence of active or significant symptoms of MG or to optimize the medical treatment. Particularly, the levels of corticosteroids should be decreased, prior to surgery. The risk of post-operative respiratory failure may be reduced by preoperative intravenous immunoglobulin administration or plasmapheresis, particularly in patients with partially-controlled symptoms (15,16).
Regarding timing for surgery, there isn’t a gold standard, but it seems that an early removal of the thymic gland may improve the remission rate (16). Age over 50 years or antibody-negative disease are relative contraindications to thymectomy in nonthymomatous MG (17,18).
Moreover, all patients should be evaluated with a contrast-enhanced CT scan of the chest. Magnetic resonance and/or PET-CT scan can also be performed in the suspicion of thymoma or to distinguish between a thymic hyperplasia and a small thymoma.
Pre-operative chest X-rays should be performed to evaluate signs of extensive adhesions, related to prior pleuritis or thoracic surgical procedure, which may preclude a robotic approach.
Functional assessment should be completed with pulmonary function tests, complete blood examination and electrocardiogram.
Pre-operative preparation
The operation is performed under general anesthesia and the patient is ventilated through a double-lumen endotracheal tube. During procedure, patients are monitored by ECG, arterial line, pulse oximeter and urine output.
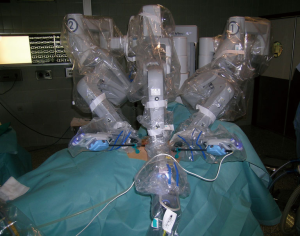
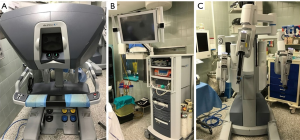
The patient is placed left or right-side up (based the side of operation), at a 30-degree angle with a bean bag. In left-sided approaches, the left arm is placed parallel to the bed while the right arm is positioned along the body to expose the axillary region (opposite for right-side operations). In the operating room the surgeon console is positioned away from the patient while the video column is at the bottom of the bed. The cart with the robotic arms is positioned on the right side of the bed at a 45° angle (opposite for right-sided approach) (Figure 1).
Because of the risks of an emergency conversion, the operative field should always be draped for an eventual median sternotomy.
Equipment preferences and cards
The most widespread surgical robot is the Da Vinci system (Intuitive Surgical, California, USA).
The Da Vinci system consists of a console where the surgeon sits while operating, connected to a patient-side cart with three interactive arms and a vision system (Figure 2).
The vision system is composed of a high definition touch-screen monitor, to allow the view of the operative field by the room staff, the video control systems and is connected to the two-channel endoscopic camera. The images captured by the 12-mm optic camera (0°–30°) are transferred to the console where the computer creates a virtual 3-D image of the operative field.
In the cart, there is also place for the CO2-supply system and its intracavitary regulation.
The patient-side cart is equipped with three robotic arms, the central one holding the camera whereas the other two are connected to the surgical instruments. The left arm is generally connected with an atraumatic grasper instrument and the right arm is equipped with a monopolar cautery or an ultrasound dissector. The surgical instruments are designed to articulate with the main arm with seven degrees of motion and a 360° rotation, which is superior to the dexterity of human hand.
The surgeon sits at the console far from the patient and through a binocular localized in the upper part of the console he is able to see a 3D image of the operative field.
The surgeon’s fingers grasp the master controls below the display and the robotic system allows the surgeon’s hands and fingers movements to be translated into identical, precise movements of the instruments inside the patient’s body. Moreover, the system filters the physiological hands’ tremor, allowing extremely precise movements.
Procedure
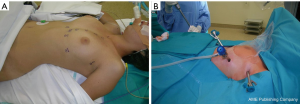
Through an incision on the fifth intercostal space on the anterior/midaxillary line, a 12 mm port for the 3-D camera is introduced. Then other two incisions are performed, one on the midaxillary region on the third intercostal space and another on the parasternal space on the fifth intercostal space and two 8 mm thoracic ports are inserted (Figure 3A). The arms of the robotic system are then connected to the ports (Figure 3B). After placing the first port, it is advantageous to use the camera to help placing the other two ports, so that their trajectory line is correct and to avoid lesion of the heart or pericardium when placing the parasternal port.
CO2 is inflated in the hemithorax through the camera port (pressure range 6–10 mmHg) to achieve a clear view within the pleural cavity and, enlarging the mediastinal space, it allows an easier dissection.
To exclude any pleural lesion in case of thymic tumors, is mandatory to perform a careful exploration of the pleural space before beginning the operation.
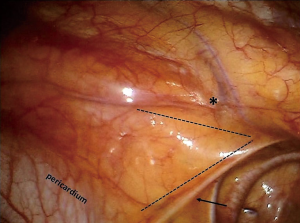
The surgical field is delimited by a triangle area with the lateral borders represented by the left or right phrenic nerves posteriorly and the mammary vessels anteriorly, the basis by the pericardium and with the apex situated at the base of the neck (Figure 4).
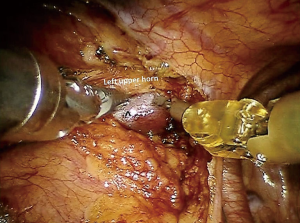
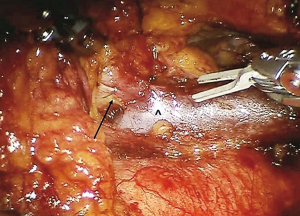
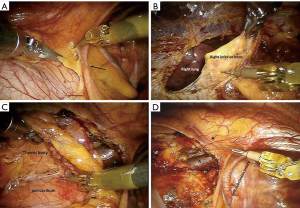
In case of left-sided approach, the dissection begins at the level of the left pericardiophrenic angle and moves upwards following the anterior border of the phrenic nerve, until all the mediastinal tissue is isolated from the nerve (Figure 5A). Caution must be paid not to damage the nerve, therefore during dissection it may be useful if the table-site assistant puts a hand on the patient abdomen to perceive any hemidiaphragm contraction. Subsequently, the thymic gland is dissected from the posterior aspect of the sternum until the right pleura is found and the right inferior horn is dissected (Figure 5B). The thymus is then mobilized upwards and separated from the pericardium, up the level of aortic arch (Figure 5C). In the superior part of the mediastinum, the pleura is opened between the phrenic nerve and the mammary vessels (Figure 5D). The dissection continues towards the base of the neck until the superior horns are found and separated by a blunt dissection from the inferior part of the thyroid (Figure 6). The superior horns are grasped and pulled downwards, the innominate vein is identified and dissected along its upper border, identifying, clipping and dividing the thymic veins (Figure 7).
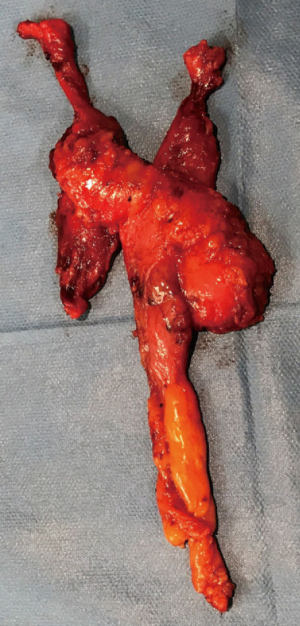
When all the thymic tissue, the mediastinal and fat tissue are radically dissected, the resected specimen (Figure 8) is placed in an endoscopic bag and removed through the medial trocar incision.
In case of a right-sided operation, dissection starts from the cardiophrenic angle and then moves upwards with the mediastinal pleura incised following the right phrenic nerve, until all anterior mediastinum is separated from the nerve. At this point the pleura is incised along the right internal mammary artery and veins, from where the vessels originate all the way down to the diaphragm, dividing the mediastinal tissue from the sternum. The next step involves the dissection of the thymus from the pericardium, beginning down at the level of the inferior horns and moving upwards where the left innominate vein is identified. The thymus is separated from the brachiocephalic vein and the thymic veins are sequentially clipped and divided. The superior horns are then bluntly dissected from the inferior portion of the thyroid. To identify the phrenic nerve on the left side, the left mediastinal pleura is then opened and then the dissection of the gland is finished.
After controlling hemostasis, a chest tube is positioned through the medial port, the lung is inflated, and the other incisions are closed.
Role of team members
Surgical team should be composed by at least 2 surgeons, 1 anesthesiologist, 1 scrub nurse and 1 operating room nurse. One surgeon is at the robotic console, controlling the robot. The other is the table-site surgeon that must perform the incisions and introduce the ports. Moreover, he must be able to perform the connection between the robot and the ports and to rapidly undock the system and perform an emergency sternotomy in case of uncontrollable vascular bleeding or any other complication. The scrub nurse must be trained in the use of robotic material and able to exchange the robotic instruments. Operating room staff must be educated on the electronics needed for robotic surgery and how to connect and calibrate the robotic system components, and solve any technical problems that may arise during procedures.
Members of the robotic surgical staff must be familiar with the da Vinci system components, as well as how to problem-solve in the event of mechanical or electrical failure.
The anesthesiologist should be experienced in the management of patients undergoing thoracic procedures and of patient with MG. Frequent matter of concern may be represented by encroachment of the anesthesia workspace by the robot and difficulty in accessing the patient intra-operatively, therefore it is required a careful arrangement of the instrumentation and room organization.
Post-operative management
If possible, extubation is performed in the operating room and then the patient may then return to the surgical thoracic ward. In some cases, depending on the anesthesiologist’s evaluation (i.e., patients with myasthenia gravis not well controlled pre-operatively) the patient can be transferred to the ICU for monitoring.
If the chest X-ray performed after operation doesn’t shows any pathological findings and the quantity of fluid from the chest drain is permissive, the chest tube is removed generally 24 hours after operation and the patient is discharged 48–72 hours after surgery.
Tips, tricks and pitfalls
Different issues regarding robotic thymectomy are still matter of debate.
First is the choice of the side to perform the operation. The choice may be guided by anatomic analysis over the anatomy of the patient’s thymus, considerations over the safety of trocars placement and based on the surgeons’ preference (19).
Authors supporting a left-sided thymectomy base their choice on the observation that the left lobe is usually bigger and extended to the cardiophrenic region, and that region below the left innominate vein and the aortopulmonary window and are frequently sites of ectopic thymus. Common possible findings are a thymic gland that extends under or lateral to the left phrenic nerve, or descends posteriorly to the brachiocephalic vein (19). Another critical point is represented by the position of the right phrenic nerve that is partially protected by the superior vena cava and may be easily identified in the lower part of the mediastinum (20,21).
Other authors prefer a right-sided thymectomy, describing an easier learning curve with this kind of approach, mainly because of a better ergonomic position to accomplish dissection and a larger operative field, due to the absence of the heart, but also an optimal visualization of the venous confluence and of the aortocaval groove (19,22,23).
Anyway, the choice of the approach should be based on the patient’s distribution of the thymic tissue, to perform a complete removal of the thymus and of all the mediastinal tissue. This is particularly important in patients with MG to obtain improvement or remission of the neurological symptoms, as the mediastinal fat is frequent site of ectopic foci of thymic tissue (24).
A particular care should be used when left innominate vein is dissected in order to avoid a major bleeding: when a small thymic vein is encountered and clipped, it is mandatory to search for a second vein that usually is present at the level of left innominate/superior vena cava angle. In about 5% to 10% of cases an anatomical variation is present: the most common is the upper left horn running behind the innominate vein and over the subclavian or carotid artery. In case of right-sided approach, the dissection of the abnormally positioned left upper horn may be demanding.
Another important point is the application of the robotic technique in patients with early stage thymoma. Historically, clinicians have been averse to use minimally invasive techniques for thymic tumors, both because of the supposed risk of rupture of the tumors’ capsule during the manipulation of the lesion with the endoscopic instruments and the possibility of reduced safety margins with an increased probability of local recurrence (25-27).
Anyway, different studies demonstrated that robotic thymectomy for early stage thymomas is a promising technique with good results both from the surgical and oncological point of views (10).
Correct selection of patients is mandatory in case of thymomas, in order to avoid complications and achieve the best result from the oncological point of view.
Some radiological criteria have been identified to guide in the selection of the better patients to propose for minimal invasive thymectomy: the tumor location in the anterior mediastinum and its encapsulation, the presence between the thymoma and the other structures of a fat plane, the absence of compression on the surrounding structures, the tumor extending on one side and the presence of normal thymic tissue (28).
It is also still matter of debate the proper size of thymomas for robotic operations. The majority of the authors consider acceptable lesions smaller than 5 cm (21,25). Larger tumor’s dimension, while not being an absolute contraindication, could make manipulation more difficult and interfere with the thoracoscopic procedure, prolonging the operative time, increasing the chance of an open conversion and the risks of tumor’s rupture and spreading (21).
Because of the increasing popularity of minimally invasive techniques, the ITMIG recently proposed some standard policies regarding thymectomy. According to common surgical oncologic principles, thymomas must be always removed together with the surrounding normal thymus and fat, in order to obtain adequate safety margins, also for capsulated tumors. Moreover, it should be used a “no-touch” technique, where the unharmed thymic tissue and perithymic fat should be used for grasping and tractioning the tumor, avoiding the rupture of the capsule and the risk of pleural implantation (29). This technique is clearly a more complicated operation because of the need of a more accurate dissection, has a prolonged learning curve with delayed operative time compared to open techniques (21).
Certainly, open conversion is mandatory if, during a minimally invasive operation, the surgeon identifies any risk for the patient or any possible violation of the oncological principles.
Robotic surgery has some major disadvantages. This type of operation is known to be highly expensive, considering the costs of the robot itself, that may be limited by the multidisciplinary use of the robot, but also the costs for the annual maintenance and of the expensive disposable robotic instruments (11,30).
Moreover, there could be an increase in the risk of damaging delicate structures because of the absence of tactile feedback. Anyway, the superior view gained by the 3-D vision seems to overcome this disadvantage (21,31).
Finally, another disadvantage is represented by the placement of the surgeon, operating at a non-sterile console away from the patient (12,31). Thus, the sterile surgeon near the robot should be able to perform alone an emergency conversion. However, this emergency procedure could be made more difficult because of the time needed for the undocking of the robotic system.
Conclusions
Nowadays, robotic thymectomy is a proven technique in many centers. Available data show that it is a feasible and safe operation, with effective and encouraging long-term results in patients with non-thymomatous myasthenia and promising outcomes both from the oncological and surgical point of view in patients affected by thymoma. Through enhanced vision and high dexterity of instruments’ movements it permits a safe and complete dissection of the thymic tissue, superior to standard thoracoscopic techniques. Robotic thymectomy is set to become the standard technique for thymectomy in patient affected by early stage thymomas and myasthenia gravis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rea F, Marulli G, Bortolotti L, et al. Experience with the "da Vinci" robotic system for thymectomy in patients with myasthenia gravis: report of 33 cases. Ann Thorac Surg 2006;81:455-9. [Crossref] [PubMed]
- Detterbeck FC, Zeeshan A. Thymoma: current diagnosis and treatment. Chin Med J (Engl) 2013;126:2186-91. [PubMed]
- Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med 2016;375:511-22. [Crossref] [PubMed]
- Balduyck B, Hendriks JM, Lauwers P, et al. Quality of life after anterior mediastinal mass resection: a prospective study comparing open with robotic-assisted thoracoscopic resection. Eur J Cardiothorac Surg 2011;39:543-8. [Crossref] [PubMed]
- Loulmet D, Carpentier A, d'Attellis N, et al. Endoscopic coronary artery bypass grafting with the aid of robotic assisted instruments. J Thorac Cardiovasc Surg 1999;118:4-10. [Crossref] [PubMed]
- Reichenspurner H, Damiano RJ, Mack M, et al. Use of the voice-controlled and computer-assisted surgical system ZEUS for endoscopic coronary artery bypass grafting. J Thorac Cardiovasc Surg 1999;118:11-6. [Crossref] [PubMed]
- Yoshino I, Hashizume M, Shimada M, et al. Thoracoscopic thymomectomy with the da Vinci computer-enhanced surgical system. J Thorac Cardiovasc Surg 2001;122:783-5. [Crossref] [PubMed]
- Ashton RC Jr, McGinnis KM, Connery CP, et al. Totally endoscopic robotic thymectomy for myasthenia gravis. Ann Thorac Surg 2003;75:569-71. [Crossref] [PubMed]
- Rea F, Bortolotti L, Girardi R, et al. Thoracoscopic thymectomy with the 'da Vinci' surgical system in patient with myasthenia gravis. Interact Cardiovasc Thorac Surg 2003;2:70-2. [Crossref] [PubMed]
- Marulli G, Maessen J, Melfi F, et al. Multi-institutional European experience of robotic thymectomy for thymoma. Ann Cardiothorac Surg 2016;5:18-25. [PubMed]
- Melfi F, Fanucchi O, Davini F, et al. Ten-year experience of mediastinal robotic surgery in a single referral centre. Eur J Cardiothorac Surg 2012;41:847-51. [Crossref] [PubMed]
- Rückert JC, Ismail M, Swierzy M, et al. Thoracoscopic thymectomy with the da Vinci robotic system for myasthenia gravis. Ann N Y Acad Sci 2008;1132:329-35. [Crossref] [PubMed]
- Melfi FM, Fanucchi O, Mussi A. Minimally invasive mediastinal surgery. Ann Cardiothorac Surg 2016;5:10-7. [PubMed]
- Pompeo E, Tacconi F, Massa R, et al. Long-term outcome of thoracoscopic extended thymectomy for nonthymomatous myasthenia gravis. Eur J Cardiothorac Surg 2009;36:164-9. [Crossref] [PubMed]
- Sardenberg RA, Abadalla RZ, Abreu IR, et al. Robotic thymectomy for myasthenia gravis. J Bras Pneumol 2011;37:694-6. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Lerut TE, et al. Thoracoscopic thymectomy in autoimmune myasthenia: results of left-sided approach. Ann Thorac Surg 2000;69:1537-41. [Crossref] [PubMed]
- Sussman J, Farrugia ME, Maddison P, et al. Myasthenia gravis: Association of British neurologists’ management guidelines. Pract Neurol 2015;15:199-206. [Crossref] [PubMed]
- Alkhawajah NM, Oger J. Treatment of myasthemia gravis in the aged. Drugs Aging 2015;32:689-97. [Crossref] [PubMed]
- Kumar A, Asaf BB. Robotic thoracic surgery: The state of the art. J Minim Access Surg 2015;11:60-7. [Crossref] [PubMed]
- Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [Crossref] [PubMed]
- Marulli G, Rea F, Melfi F, et al. Robot-aided thoracoscopic thymectomy for early-stage thymoma: a multicenter European study. J Thorac Cardiovasc Surg 2012;144:1125-30. [Crossref] [PubMed]
- Deen S, Farivar AS, Louie BE. Thoracic techniques: robotic thymectomy for thymoma. Indian J Surg Oncol 2013;4:132-7. [Crossref] [PubMed]
- Nakamura H, Taniguchi Y. Robot-assisted thoracoscopic surgery: current status and prospects. Gen Thorac Cardiovasc Surg 2013;61:127-32. [Crossref] [PubMed]
- Zieliński M, Kuzdzal J, Szlubowski A, et al. Comparison of late results of basic transsternal and extended transsternal thymectomies in the treatment of myasthenia gravis. Ann Thorac Surg 2004;78:253-8. [Crossref] [PubMed]
- Toker A, Erus S, Ozkan B, et al. Does a relationship exist between the number of thoracoscopic thymectomies performed and the learning curve for thoracoscopic resection of thymoma in patients with myasthenia gravis? Interact Cardiovasc Thorac Surg 2011;12:152-5. [Crossref] [PubMed]
- Ye B, Tantai JC, Li W, et al. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery in the surgical treatment of Masaoka stage I thymoma. World J Surg Oncol 2013;11:157. [Crossref] [PubMed]
- Friedant AJ, Handorf EA, Su S, et al. Minimally Invasive versus Open Thymectomy for Thymic Malignancies: Systematic Review and Meta-Analysis. J Thorac Oncol 2016;11:30-8. [Crossref] [PubMed]
- Cheng YJ, Hsu JS, Kao EL. Characteristics of thymoma successfully resected by videothoracoscopic surgery. Surg Today 2007;37:192-6. [Crossref] [PubMed]
- Toker A, Sonett J, Zielinski M, et al. Standard terms, definitions, and policies for minimally invasive resection of thymoma. J Thorac Oncol 2011;6:S1739-S1742. [Crossref] [PubMed]
- Augustin F, Schmid T, Sieb M, et al. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery thymectomy. Ann Thorac Surg 2008;85:S768-S771. [Crossref] [PubMed]
- Weksler B, Tavares J, Newhook TE, et al. Robot-assisted thymectomy is superior to transsternal thymectomy. Surg Endosc 2012;26:261-6. [Crossref] [PubMed]
Cite this article as: Marulli G, Comacchio GM, Rea F. Robotic thymectomy. J Vis Surg 2017;3:68.