Video-assisted thoracoscopic surgery training with a polyvinyl-alcohol hydrogel model mimicking real tissue
Introduction
More than two decades have passed since the initial introduction of anatomic resection using video-assisted thoracoscopic surgery (VATS) in the 1990s, and this technique has become widely accepted in the clinical field. The American Board of Thoracic Surgery requires surgical trainees to have experience in minimally invasive thoracic surgery (including VATS and robotics). In Japan, board certification by the Japanese Association for Chest Surgery requires participation in a VATS training course consisting of basic dry box training and wet-lab training using an anesthetized porcine model. In order to support the learning of this comparatively difficult technique, various training methods and simulators have been developed, including cadaveric training, the use of extracted porcine or bovine cardiopulmonary tissue blocks, and virtual reality simulators (1-3). In this report, we introduce VATS training for lobectomy using a three-dimensional modeled rib cage accommodating a non-biological lung model that provides an anatomical structure and texture close to that of a human.
Training materials and methods
Three-dimensional rib cage model and polyvinyl-alcohol (PVA) hydrogel lung model
A skeletonized human rib cage model with bony ribs was developed by Morikawa and his collaborators (BiotextureTM, FASOTEC Co., Ltd. Chiba, Japan) (Figure 1). The details of this thoracic cage and PVA hydrogel lung model are described in a recently published manuscript (4). This skeletonized model can accommodate PVA hydrogel lung models that are designed to be replaced after each training session. A prepared silicon-based ‘skin’ flap covers the model in which the trainees can introduce incision for ports or access windows.
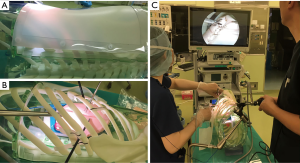
The PVA lung model (BiotextureTM) comprises water-containing PVA, which realizes a texture that is comparable to the real organ. Because this foamed PVA model is electro-conductive, it not only simulates real texture, but also enables the use of electrocautery or energy devices. This model comprises shrunken lung parenchyma, pulmonary arteries, veins, bronchi, nerves, lymph nodes, connective tissue, and membranes such as pleura.
Lobectomy and lymph node dissection using the PVA model in the BiotextureTM rib cage
Demonstrations of right lower lobe lobectomy and subsequent right upper lobe lobectomy are shown in Figures 2,3 respectively. Figure 4 presents a mediastinal lymph node dissection following the lobectomies in this model.



Training of VATS segmentectomy
Training session records of left upper lobe lobectomy (superior segmentectomy) are shown in Figures 5,6. During the procedures, the trainee was able to see through to the inside of the chest cavity to check and understand the optimized direction of the instruments or staplers. In Figure 6, the trainee and the instructor interrupted the procedure and pulled out the model from the rib cage to examine the three-dimensional structure of the vessels and the bronchus. Note that this model provided lung parenchyma that can be resected by electrocautery and energy devices, and also presented an accurate three-dimensional structure that included the division of vessels and bronchi allowing for realistic segmentectomy training.


Discussion
The advantages of VATS over thoracotomy (including reduced perioperative complications and shorter length of stay with equivalent oncologic outcomes) have been recognized for years. Accordingly, the number of VATS lobectomy procedures has doubled in the past decade; approximately 36% of lobectomies in Japan were performed using VATS in 2005, and recent statistics have shown that the figure doubled in 2015 (10,11). Thus, VATS procedures have become a de facto necessary skill for thoracic surgeons to learn.
In pulmonary resections, comprehensive understanding of the anatomical structures of pulmonary arteries, veins, and bronchi is essential. These networks intersect each other in a complicated manner, and incomplete fissure may further obscure these structural relationships. In the thoracoscopic setting, the placement, scope, and movement of the instruments are limited because of the patient’s hard bony rib cage, which restricts the field of view and perspective. Therefore, training with a simulator that provides a bony rib cage is ideal for addressing these issues.
Training systems that use living animals have numerous advantages. Pulsation and the movement of the mediastinum driven by respiration provide realistic sensations. Trainees can also gain experience in tissue incision, dissection, coagulation hemostasis using electrocautery, energy devices, ultrasound devices, as well as actual procedures. In addition, they can learn how to prevent and manage injuries to the vessels and lung parenchyma. The major drawback of animal-based training is the fundamental anatomical difference from humans; in porcine models, the left-side anatomy relatively closely mirrors that of humans, but the procedures in the shallow and narrow thoracic space are different from when they are conducted in humans. Tissue simulator-based training using extracted lung and heart blocks has the advantage of easy availability from slaughterhouses at relatively low cost, and training using these tissue blocks can be conducted in a wide variety of settings. There have been several reports demonstrating the effectiveness of these types of simulators (3,12). However, they require special instruments that account for the heightened risk of contamination and infection. Moreover, it is difficult to introduce global standards due to religious and ethical issues.
To address these concerns, Iwasaki and colleagues developed a VATS training method using a dry box with an anatomically correct model in the early stages of VATS procedures (2). They designed a lung model consisting of lung parenchyma, pulmonary arteries, veins, and bronchi made with foamed polyurethane. Despite the relative simplicity of their model, it provided the basic anatomical structures and allowed for optimal results in the evaluation of medical students’ understanding of lung anatomy (13). The PVA hydrogel model developed by Morikawa and colleagues should be regarded as an evolutionary stage of the polyurethane model mentioned above.
With the use of modeled rib cages, the PVA model is equipped with more realistic structure and texture than the polyurethane model, and has reached a level that can satisfy the needs of senior trainees or even experienced surgeons. Furthermore, the non-biological lung model is free from the risk of contamination or infection, and instruments employed in daily clinical use in the operation theater can be used. This aspect allows training to be conducted in the actual surgical settings, and also reduces costs because there are no requirements for special instruments or space.
The disadvantage of this biomimicking PVA model simulator is its expense: the unilateral lung model costs approximately US$500. At its current cost, it may be too expensive for casual practice when considering that an extracted porcine heart-lung tissue block costs only US$100. Although there remains room for improvement to achieve more realistic texture, this model should be regarded as an effective simulator and training system at present.
Acknowledgements
A subsidy for manufacturing business services was provided to FASOTEC Co., Ltd. from the Ministry of Economy, Trade and Industry of Japan.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- Jensen K, Ringsted C, Hansen HJ, et al. Simulation-based training for thoracoscopic lobectomy: a randomized controlled trial: virtual-reality versus black-box simulation. Surg Endosc 2014;28:1821-9. [Crossref] [PubMed]
- Iwasaki A, Moriyama S, Shirakusa T. New trainer for video-assisted thoracic surgery lobectomy. Thorac Cardiovasc Surg 2008;56:32-6. [Crossref] [PubMed]
- Meyerson SL, LoCascio F, Balderson SS, et al. An inexpensive, reproducible tissue simulator for teaching thoracoscopic lobectomy. Ann Thorac Surg 2010;89:594-7. [Crossref] [PubMed]
- Morikawa T, Y.M., Odaka M, Tsukamoto Y, et al. A Step-by-Step Development of Real-Size Chest Model for Simulation of Thoracoscopic Surgery. Interactive Cardiovascular and Thoracic Surgery 2017. [Epub ahead of print].
- Sato T, Morikawa T. Right lower lobe lobectomy. Asvide 2017;4:184. Available online: http://www.asvide.com/articles/1492
- Sato T, Morikawa T. Right upper lobe lobectomy following the right lower lobe lobectomy. Asvide 2017;4:185. Available online: http://www.asvide.com/articles/1493
- Sato T, Morikawa T. Mediastinal lymph node dissections. Asvide 2017;4:186. Available online: http://www.asvide.com/articles/1494
- Sato T, Morikawa T. The left upper lobe lobectomy (apico-posterior and anterior segmentectomy) performed by a trainee and an instructor. Asvide 2017;4:187. Available online: http://www.asvide.com/articles/1495
- Sato T, Morikawa T. The training was interrupted to allow the trainee to comprehend the anatomy of the bronchi and vessels before completing the intersegmental plane. Asvide 2017;4:188. Available online: http://www.asvide.com/articles/1496
- Committee for Scientific Affairs. Thoracic and cardiovascular surgery in Japan during 2013: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2015;63:670-701. [Crossref] [PubMed]
- Ueda Y, Osada H, Osugi H, et al. Thoracic and cardiovascular surgery in Japan during 2005. Annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2007;55:377-99. [Crossref] [PubMed]
- Tong BC, Gustafson MR, Balderson SS, et al. Validation of a thoracoscopic lobectomy simulator. Eur J Cardiothorac Surg 2012;42:364-9;discussion 369. [Crossref] [PubMed]
- Obuchi T, Imakiire T, Hamatake D, et al. Efficacy of simulated surgery using anatomically correct lung models for trainees to learn major resections. The Journal of the Japanese Association for Chest Surgery 2011;25:103-6. [Crossref]
Cite this article as: Sato T, Morikawa T. Video-assisted thoracoscopic surgery training with a polyvinyl-alcohol hydrogel model mimicking real tissue. J Vis Surg 2017;3:65.


