Laparoscopic techniques and strategies for gastrointestinal GISTs
Introduction
Laparoscopic surgery has been widely accepted in all surgical fields, since laparoscopic cholecystectomy was introduced in the late 1980s (1) Surgical treatment for gastrointestinal submucosal tumor (SMT) has not been excluded from this trend. Laparoscopic wedge resection has been a widely accepted procedure for SMTs of stomach (2). Additionally, laparoscopic surgery has been feasibly performed for SMTs of small bowel, even though this procedure is rarely applied due to the low prevalence (3).
However, as we perform laparoscopic surgery for an SMT of gastrointestinal tract, the possibility of gastrointestinal stromal tumor (GIST) should be considered at all times. GISTs sometimes show malignant behavior and account for a considerable portion of gastrointestinal SMTs. Therefore, surgeons should focus on certain principles when laparoscopic surgery is used for SMTs presenting with features of GISTs, even though GISTs are mostly confirmed by postoperative pathologic examination. In this chapter, we will introduce laparoscopic techniques and strategy for gastrointestinal SMTs, in accordance with those for GISTs.
Patient selection and work up
Patient selection
The indication for laparoscopic approach has been related to tumor size. In case of gastric SMTs, the limitation of tumor size for laparoscopic surgery has traditionally been considered “diameter not more than 2 cm,” but it has become larger based on the recent evidences. In 2006, Otani et al. (4) reported that laparoscopic surgery had been feasibly performed for SMTs up to 5 cm in diameter. Moreover, some researchers also reported their experiences of laparoscopic surgery for larger SMTs (>5 cm, but <10 cm) (5,6). This limit regarding tumor size is expected to be higher in the near future. With respect to this issue, the current guidelines do not state an absolute indication regarding tumor size (7,8). In addition, there is no lower limit on tumor size. Actually, in our institute, the indications for laparoscopic surgery include the relatively small SMT (<10 mm in diameter and definitely visible by endoscopy), regardless of symptoms (9).
Meanwhile, with regard to SMTs at small bowels, a size limit for laparoscopic approach has not been established. Unlike the stomach, as these organs are mainly composed of tubular structures, laparoscopic manipulation can be easily performed (10). For example, even if we encounter a large jejunal or ileal tumor, this condition may be controlled by manipulating the nearby segments. However, duodenal SMTs require some considerations regarding tumor size, since the 2nd portion of duodenum correlates with the surrounding structures (i.e., biliary or pancreatic duct).
Work up of the selected patient
All patients undergo preoperative examinations to clarify the clinical diagnosis. Preoperative data include the tumor size, location, growth pattern, and the presence of metastasis is determined through the combined evaluation using gastrofiberscopy, upper gastrointestinal radiography, endoscopic ultrasonography (EUS), and computed tomography (CT). Endoscopic biopsy or EUS is performed only when clinically indicated.
Pre-operative preparation
Before operation, tumors <2 cm in diameter measured on CT or EUS are localized by gastrofiberscopic clipping of the mucosa covering the SMT. With respect to small gastric SMTs, the general information regarding tumor characteristics (e.g., location and shape of tumor) are intuitively provided by CT gastrography, in which virtual gastroscopy and surface-shaded volume rendering techniques are included (9).
After general anesthesia is induced, the patient is placed in the supine position, with his or her legs abducted. At first, a scope port is established on the umbilical area, and pneumoperitoneum is made by carbon dioxide infused through the scope port, with an insufflation pressure of 12 mmHg. Then, a flexible or 30-degree rigid scope is inserted through this port. After we performed diagnostic laparoscopy, two ports are additionally established on the upper abdomen to allow identification of the tumor location and to apply a laparoscopic linear cutter. The operator surgeon is usually positioned on the right side of the patient, with the scopist positioned between the patient’s legs, managing the scope. An assistant surgeon can be additionally positioned on the left side of patient.
Procedure
In general, the principle of surgical treatment for GISTs is local resection with a negative resection margin (2). Laparoscopic surgery is no exception to this general principle. In addition, according to the location and configuration (i.e., exophytic versus endophytic) of tumor, the different surgical techniques are requested.
Anterior wall of stomach
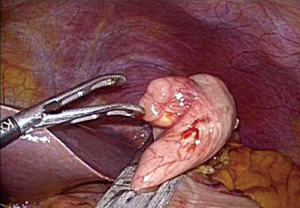
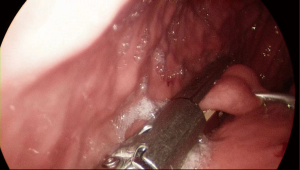
Exogastric approach is predominantly applied for exophytic tumors at the gastric anterior wall (2). In this condition, we can isolate the tumor by manipulating the surrounding normal tissue with laparoscopic instruments (11). Then, to acquire the oncologic safety margin, laparoscopic linear stapler should be applied to the normal tissue of stomach (Figure 1). If the gastric wall is excessively resected, this procedure can result in deformity or stenosis of the gastric lumen (2). Thus, laparoscopic linear stapler should be applied perpendicularly to the longitudinal axis of the stomach (11).
Recently, eversion method, another technique to prevent stenosis, has been introduced (12). When a large endophytic SMT is located at the gastric anterior wall, it is difficult to trace the tumor in the serosal surface. In this situation, eversion method involves a gastrotomy, through which the tumor can be everted out of the stomach. Then, laparoscopic linear stapler is applied to the stalk of the everted tumor. Simultaneously, the gastrotomy can be closed with the linear cutter. At this time, the gastrostomy should be made near the point at which the tumor is expected to locate. In addition, as for exogastric approach, the direction of laparoscopic stapler should also be perpendicularly arranged to the longitudinal axis of the stomach.
Posterior wall of stomach
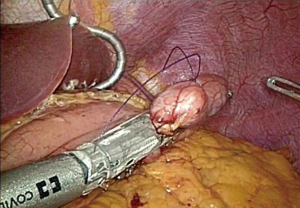
If SMTs are located at the gastric posterior wall, the appropriate approach is adopted according to whether the tumor is exophytic or endophytic. If it is possible to mobilize and rotate the stomach, the exogastric approach can be applied for an exophytic lesion (Figure 2) (13). However, an endophytic lesion mostly requires the more complex approaches, since the tumor localization is not easy during laparoscopic surgery. In general, either transgastric or intragastric approach can be applied.
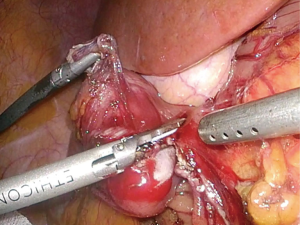
As shown in Figure 3, transgastric approach is to resect the tumor through an anterior gastrotomy (14). This gastrotomy can be closed by either hand-sewing or stapling (The direction of stapling line should be arranged perpendicularly to the longitudinal axis of the stomach).
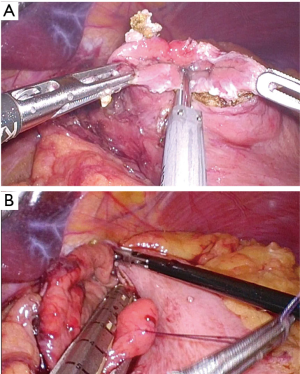
The intragastric approach is to resect the tumor with laparoscope and instruments inserted into the gastric lumen (Figure 4) (15). In order to prevent the retraction of trocar, balloon trocar is recommended for use in this method (16). Recently, several surgeons reported single-incision intragastric approach (15). In this procedure, the stomach is brought out and incised through a single umbilical incision, which is kept open with a wound retractor. Then, the tumor is manipulated and resected via a commercial 4-holes single port inserted through the gastrotomy.
Lesser curvature/prepyloric antrum of stomach
When SMTs are located at the lesser curvature or prepyloric antrum, laparoscopic wedge resection is challenging procedures (2). As these areas characterized by the low distensibility, postoperative stenosis of the gastric lumen may occur. In addition, there is a risk of vagus nerve injury, which can induce delayed gastric emptying or functional gastric outlet obstruction (2,11). With these reasons, many surgeons prefer full-thickness resection of the tumor without laparoscopic stapler (2). In this method, gastric wall should be closed by hand sewing. Distal gastrectomy can be considered unless stenosis or deformity can be avoided.
Esophago-gastric junction (EGJ)
Laparoscopic surgery for SMTs at EGJ is also technically demanding procedure. If the lesion shows a malignant potential, invasive approach is sometimes required (2). In other words, radical surgery is rather safer than wedge resection, because the attempt to get an adequate resection margin can result in stenosis of EGJ. With this reason, in several institutes, total gastrectomy has been performed for EGJ tumors. Even if laparoscopic proximal gastrectomy was introduced in 1990s (17), most surgeons have avoided this procedure on account of late complications, such as reflux esophagitis or stricture, regarding esophago-gastrostomy (2). Double tract reconstruction is one of the potential solutions for this issue. Recently, Ahn et al. (18) reported that less reflux and stricture are showed after laparoscopic proximal gastrectomy with double tract reconstruction.
Nevertheless, for most SMTs including GISTs, proximal gastrectomy is also excessive procedure. Intragastric or transgastric approach is a useful option for benign or even some malignant lesions (Figure 5). In addition, for certain benign lesion, laparoscopic enucleation can be a good solution to prevent stenosis of EGJ (20).

Small bowel
Meanwhile, SMTs of small bowel is resected extracorporeally or intracorporeally based on the surgeon’s preference. When wedge resection is tried for a duodenal turmor (Figure 6), it is important not to involve the papillary region. In addition, gastrojejunostomy is often necessary, as duodenum is also risk of postoperative stenosis. In case of jejunal of ileal SMT, extracorporeal resection is easily performed through an umbilical port wound even though the tumor is large (3,11). However, it is important to avoid the iatrogenic spillage of tumor cells during the whole time of laparoscopic surgery. In addition, when the tumor invaded to the adjacent organ, conversion to open surgery should be considered.
Completion of laparoscopic surgery
After resection of tumor, the specimen is placed in a vinyl bag, which is delivered through the umbilical port wound that is slightly enlarged to accommodate the tumor. After hemostasis is achieved and integrity of the staple line is confirmed, the abdominal cavity is irrigated and the port wounds are closed.
Post-operative management
If the patient undergoes wedge resection, a semi-bland diet will be provided within 48–72 hours. However, in cases involving proximal or distal gastrectomy, the diet will be restricted for several days.
Whether adjuvant chemotherapy is administered should be determined according to the postoperative pathologic report, in which the final diagnosis is also revealed. The final pathologic report includes the immunohistochemical staining profile, tumor size, tumor mitotic index, and resection margin status. The lesions in which the postoperative immunohistochemical staining is positive for CD117 (KIT) are diagnosed as GISTs, and mutation analysis for the c-KIT mutation is also performed (9). GISTs are divided into four groups according to the Risk Assessment Classification proposed by Fletcher et al. (21). Mitotic figures for all specimens are counted in 50 randomly selected high-power fields by the experienced pathologist.
Tips, tricks and pitfalls
As some GISTs carry malignant potential, we should always consider the possibility of intraperitoneal dissemination caused by manipulation of the tumor during laparoscopic approach (11). In most SMTs with benign features (i.e., lipomas leiomyomas, schwannomas, and granular cell tumors), laparoscopic approach cause little concern regarding peritoneal dissemination because those tumors seldom recur (22,23). However, since some SMTs are diagnosed as GISTs, there is a risk of high grade malignancy. Although most GISTs show an acceptable prognosis after laparoscopic surgery (24), such a favorable outcome cannot be expected in the patient who undergoing tumor rupture.
With regard to this issue, many surgeons take ‘tumor size’ into consideration, with an assumption that large tumors may require more manipulation than small, increasing the opportunity for dissemination. With this reason, laparoscopic surgery has been generally recommended for relatively small GISTs (i.e., diameter ≤2 cm) based on the GIST Consensus Conference in 2004 (25). However, the tumor size is not the only factor to decide the applicability of laparoscopic procedure. Regardless of size, open surgery may be necessary in highly vascular or fragile tumors, due to increased risk of tumor bleeding or rupture. Therefore, during laparoscopic surgery for SMT, it is desirable to handle the tumor without grasping it directly. This principle has been widely accepted, and we currently have several tips for manipulating the tumor without touching it. A “no-touch” technique, described in 2002, includes grasping the surrounding tissues, holding the threads sutured at the normal serosa around the tumor, and using a laparoscopic stapler or vinyl bag (26). By applying these techniques in laparoscopic surgery, we can minimize the risk of tumor cell dissemination.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dubois F, Berthelot G, Levard H. Cholecystectomy by coelioscopy. Presse Med 1989;18:980-2. [PubMed]
- Hwang SH, Park DJ, Kim YH, et al. Laparoscopic surgery for submucosal tumors located at the esophagogastric junction and the prepylorus. Surg Endosc 2009;23:1980-7. [Crossref] [PubMed]
- Lucas LC, Fass R, Krouse RS. Laparoscopic resection of a small bowel lipoma with incidental intussusception. JSLS 2010;14:615-8. [Crossref] [PubMed]
- Otani Y, Furukawa T, Yoshida M, et al. Operative indications for relatively small (2-5 cm) gastrointestinal stromal tumor of the stomach based on analysis of 60 operated cases. Surgery 2006;139:484-92. [Crossref] [PubMed]
- Sokolich J, Galanopoulos C, Dunn E, et al. Expanding the indications for laparoscopic gastric resection for gastrointestinal stromal tumors. JSLS 2009;13:165-9. [PubMed]
- Karakousis GC, Singer S, Zheng J, et al. Laparoscopic versus open gastric resections for primary gastrointestinal stromal tumors (GISTs): a size-matched comparison. Ann Surg Oncol 2011;18:1599-605. [Crossref] [PubMed]
- Kang YK, Kang HJ, Kim KM, et al. Clinical practice guideline for accurate diagnosis and effective treatment of gastrointestinal stromal tumor in Korea. Cancer Res Treat 2012;44:85-96. [Crossref] [PubMed]
- ESMO / European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii49-55. [PubMed]
- Ryu KJ, Jung SR, Choi JS, et al. Laparoscopic resection of small gastric submucosal tumors. Surg Endosc 2011;25:271-7. [Crossref] [PubMed]
- Lee CM, Kim HH. Minimally invasive surgery for submucosal (subepithelial) tumors of the stomach. World J Gastroenterol 2014;20:13035-43. [Crossref] [PubMed]
- Kong SH, Yang HK. Surgical treatment of gastric gastrointestinal stromal tumor. J Gastric Cancer 2013;13:3-18. [Crossref] [PubMed]
- Hyung WJ, Lim JS, Cheong JH, et al. Laparoscopic resection of a huge intraluminal gastric submucosal tumor located in the anterior wall: eversion method. J Surg Oncol 2005;89:95-8. [Crossref] [PubMed]
- Kakeji Y, Nakanoko T, Yoshida R, et al. Laparoscopic resection for gastrointestinal stromal tumors in the stomach. Surg Today 2012;42:554-8. [Crossref] [PubMed]
- Watson DI, Game PA, Devitt PG. Laparoscopic resection of benign tumors of the posterior gastric wall. Surg Endosc 1996;10:540-1. [Crossref] [PubMed]
- Na JU, Lee SI, Noh SM. The single incision laparoscopic intragastric wedge resection of gastric submucosal tumor. J Gastric Cancer 2011;11:225-9. [Crossref] [PubMed]
- Sahm M, Pross M, Lippert H. Intraluminal resection of gastric tumors using intragastric trocar technique. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2011;21:e169-72. [Crossref] [PubMed]
- Uyama I, Ogiwara H, Takahara T, et al. Laparoscopic and minilaparotomy proximal gastrectomy and esophagogastrostomy: technique and case report. Surg Laparosc Endosc 1995;5:487-91. [PubMed]
- Ahn SH, Jung DH, Son SY, et al. Laparoscopic double-tract proximal gastrectomy for proximal early gastric cancer. Gastric Cancer 2014;17:562-70. [Crossref] [PubMed]
- Lee CM, Park S. Laparoscopic techniques and strategies for gastrointestinal GISTs. Asvide 2017;4:189. Available online: http://www.asvide.com/articles/1497
- Taniguchi E, Kamiike W, Yamanishi H, et al. Laparoscopic intragastric surgery for gastric leiomyoma. Surg Endosc 1997;11:287-9. [Crossref] [PubMed]
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. [Crossref] [PubMed]
- Palazzo L, Landi B, Cellier C, et al. Endosonographic features of esophageal granular cell tumors. Endoscopy 1997;29:850-3. [Crossref] [PubMed]
- Fernandez MJ, Davis RP, Nora PF. Gastrointestinal lipomas. Arch Surg 1983;118:1081-3. [Crossref] [PubMed]
- Novitsky YW, Kercher KW, Sing RF, et al. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg 2006;243:738-45;discussion 745-7. [Crossref] [PubMed]
- Blay JY, Bonvalot S, Casali P, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol 2005;16:566-78. [Crossref] [PubMed]
- Yahchouchy-Chouillard E, Etienne JC, Fagniez PL, et al. A new "no-touch" technique for the laparoscopic treatment of gastric stromal tumors. Surg Endosc 2002;16:962-4. [Crossref] [PubMed]
Cite this article as: Lee CM, Park S. Laparoscopic techniques and strategies for gastrointestinal GISTs. J Vis Surg 2017;3:62.