Beyond the limits, extreme minimally invasive surgery in invasive thymic tumours
Introduction
Generally accepted indications for minimally invasive surgery (MIS) to thymic tumours include small, well encapsulated non-invasive tumours. MIS for more advanced complicated thymic tumours remain controversial. This is due to concerns on whether an oncologically complete resection can be done through MIS without risking tumour seedling from capsular breach during surgical manipulation. However with increased experience, MIS can be used safely in a selected group of complex thymic tumours without oncological compromise. This includes large tumours, invasive tumours, cystic thymomas, re-thymectomy in previous sternotomy and resection of tumours following neoadjuvant treatment (1-4).
Types of minimally invasive surgical approaches to thymic tumours
- Robotic assisted thoracoscopic surgery (RATS);
- Video assisted thoracoscopic surgery (VATS);
- Unilateral vs. bilateral approach;
- Single incision vs. multi-ports;
- Thoracic vs. subxiphoid;
Whichever method is employed the principles of MIS for thymic tumours especially in thymoma should be a “No touch, tumour last dissection technique” (2,3).
Surgical technique
My preference for VATS thymectomy/thymic tumours is 3-port VATS approach. It is performed under general anaesthesia with single lung isolation obtained using a left sided double lumen endotracheal tube supplemented with carbon dioxide insufflation (5–8 mmHg pressure at 4 L/min flow rate) for rapid initial deflation of the lung and opening of tissue planes for easy access to the superior horns in the neck. The patient is positioned at 30 degrees in a semi-supine position with a roll placed under the shoulder with the ipsilateral arm held abducted over a padded L-screen for exposure of the axilla (Figure 1A). However I have found that abducting the arm narrows the thoracic inlet limiting exposure to the cervical part of the thymus. Therefore recently the positioning has been modified to just placing the arm adducted to the side of the patient as in robotic thymectomy (Figure 1B). Though some surgeons use the lateral decubitus position the supine position is ideal as it gives better access to the opposite pleura/phrenic nerve, the thoracic inlet and the innominate vein. It also allows for quick conversion to a sternotomy if necessary especially in innominate vein injuries. The lateral decubitus position is useful in patients with breast implants and very large breast.
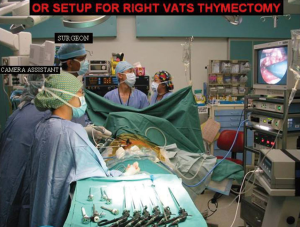
The surgeon and the camera assistant stands in front of the patient whilst the second assistant stands opposite the surgeon (Figure 2). The right side is my preferred approach for non thymomatous myasthenia gravis especially in small size females and children where there is more room for manipulation with the heart out of the way. Besides it gives better visualization of the innominate vein, superior vena cava junction. The left sided approach is used whenever the predominant tumour is located on that side.
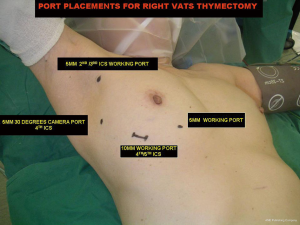
Two or three 5-mm and one 10-mm ports are inserted. Though in my initial experience 3 ports (2 ×5 mm and 1 ×10 mm) were used, with the additional 4th medial most 5 mm working/retraction port has improved exposure and quicken the surgery especially in hyperplastic thymic glands, large and invasive thymic tumours. A 30 degrees angled camera was placed in the lateral most 5-mm port at the anterior axillary line, the other ports being working ports (Figure 3). The 30 degrees lens is ideal for visualizing the opposite pleura and phrenic nerve .The right mediastinal pleura is first opened and the right phrenic nerve is identified and preserved. The thymus and its perithymic fat are mobilized from neck to diaphragm and phrenic nerve to phrenic nerve. Superior horns are dissected from the innominate vein first before the body and inferior horns of the thymus is mobilized. This is to prevent the weight of the gland from obstructing the surgical field of vision which makes subsequent dissection of the gland from the innominate vein difficult. All horns are dissected right up into the neck and delivered into the chest. This is achieved by directing the 30 degrees camera up into the chest inlet with the assistant’s finger pressing on the suprasternal notch. Accessory horns usually under the innominate vein should be routinely looked for in patients with myasthenia gravis. Identification and prevention of injury to both phrenic nerves is mandatory to ensure safe complete radical thymectomy especially in patients with myasthenia gravis. In unilateral approach the thymus is dissected right up to the opposite phrenic nerve which is identified by opening the contralateral pleura and retraction of the opposite lung by the 2nd assistant through the medial most 5 mm port (1,2). Sometimes to further improve visualization of the opposite phrenic nerve, perithymic and aortopulmonary window fats, intermittent ventilation of the opposite lung is done. Thermal injury to the nerves can be minimized by using an energy source like the bipolar dissector or ultrasonic harmonic scalpel (5). The routine use of energy source also allows for quick dissection and safe coagulation of the feeding thymic vessels. The specimen is removed by appropriate enlargement of the 10 mm port and through which a chest tube is placed. As both pleural cavities are opened functioning as one compartment, 1 chest tube suffices for drainage (5,6).
VATS thymectomy for thymomas
VATS thymectomy for thymomas was initially confined to small less than 2 cm well encapsulated intrathymic thymomas. Larger tumours were not subjected to VATS due to oncological concerns of possible risks of breach of tumour capsule with tumour seedling, port site tumour implantation and possible iatrogenic capsular injury during extraction of larger specimens making subsequent histological analysis inaccurate.
However with increased experience in VATS thymectomy, larger well encapsulated tumours were resected by modifying the VATS technique used for standard non thymomatous myasthenia gravis. Indications were extended to larger thymomas by modifying the standard VATS technique incorporating oncological principles naming it the “no touch tumour last” technique (2,3,5). The principles of this technique are:
- The side of the approach is determined by site of tumour location. This is to ensure safe dissection of the tumour under direct vision to minimize tumour capsular injury.
- Though some surgeons only dissect the tumour with adequate surrounding surgical margins and leave the rest of the thymus behind, it is always best to do a radical en bloc removal of the whole thymus with the tumour and perithymic fat. This allows for the non tumourous part of the thymus and perithymic fat to be dissected first so as to be used for grasping and traction when dissecting the tumour last. Furthermore radical complete thymectomy is paramount if the thymoma is associated with myasthenia gravis (MG) as long term neurological outcomes can be adversely affected by incomplete thymectomy. Even with thymomas with no MG radical thymectomy is always done as 10% of patients can develop MG postoperatively. Radicality of thymectomy also ensures a more oncologically complete R0 resection with adequate clear margins and prevent recurrence in remnant thymus from multifocal cancer.
- The tumour is always dissected last. This is to minimize tumour handling and manipulation. If the tumour is dissected first, the weight of the tumour under gravity will obstruct the surgical field of vision and make subsequent dissection of the gland difficult and oncologically hazardous. However when the tumour is dissected last, the non tumourous horns and thymic fat can be used for traction and grasping and not the tumour thus minimizing risk of capsular damage.
- Prevention of injury to both phrenic nerves is ensured by careful dissection under direct vision. One phrenic nerve can be sacrificed if involved by cancer.
- All specimens are removed via an Endobag by appropriate enlargement of the 10 mm port incision to prevent capsular damage. Through this incision a chest tube is placed at the end of the surgery.
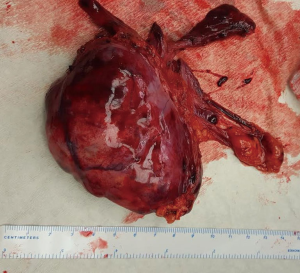
Figure 4 illustrates this principle in a 29-year-old lady with generalized myasthenia gravis. Histology showed a 9 cm × 7 cm × 3 cm WHO type B2, stage 1 Masaoka thymoma (Figure 5).

VATS thymectomy for invasive thymomas
Invasive thymomas are generally not resected by VATS due to technical difficulties and possible oncological compromise from tumour seedling. The majority of stage 3 invasive thymomas can be excluded for VATS by careful preoperative computed tomography screening for major local invasion into vital structures. However, with experience and modifications in VATS technique, selected invasive tumors can be safely resected by VATS without resorting to sternotomy (4). Surgery involves en bloc removal of tumour with thymus, perithymic fat and involved surrounding structures which may include the phrenic nerve, pericardium, lung and innominate vein (4) (Figure 6). And Figure 7 shows a bilateral VATS approach for Masaoka Stage 3, 6 cm × 6 cm WHO Type B2/B3 invasive thymoma with bilateral pericardial involvement.

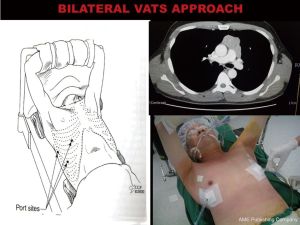
Bilateral VATS hybrid approach for thymomas and anterior mediastinal tumours
As described above well encapsulated thymomas less than 5 cm and away from the innominate vein can be resected by Unilateral VATS approach. However tumours sitting on or possible invasion of the innominate vein and large (10 cm) noninvasive tumours particularly cystic thymomas and teratomas can be challenging with unilateral VATS approach due to risks of venous and tumour capsular injuries. Unilateral approach to large mediastinal tumours especially those arising from the left side in small size patients can be challenging as the chest cavity is small with limited space for manipulation and dissection with risk of tumour capsular injury. In tumours abutting or possible invasion of the innominate vein, unilateral approach from the same side of tumour prevents visualization and access of the innominate vein as the main tumour obstructs the vein with possible risk of major vascular injury.
Since 2012 by adopting a modified bilateral VATS approach a selected sub group of these tumours were safely resected by VATS without resorting to sternotomy. A bilateral VATS hybrid approach was used incorporating the principles of the “No touch, tumour last” technique. However unlike in unilateral VATS thymomectomy where the tumour is always approached from the side of the tumour here it is approached from the opposite side first. The port placements are as in a standard unilateral VATS thymectomy. This approach enables safe dissection of the uninvolved normal thymus first followed by early identification of the innominate vein. By approaching from the opposite side first the innominate vein not involved by tumour can be identified and its usually large venous supply can be safely ligated early before the rest of the tumour is mobilized. With progressive dissection from its bed, the tumour and thymus gradually falls under gravity into the opposite chest. Once mobilization has been completed from the contralateral side a chest tube is placed through the 10 mm port and the lung is then re-expanded. After which the ipsilateral lung is isolated and the tumour is extracted from the ipsilateral chest with minimum dissection by an appropriate sub-mammary utility incision (Figures 8 and 9). Two patients with large non-seminomatous germ cell tumours were resected after adjuvant chemotherapy by this technique. One is a 21-year-old male with 21 cm × 18 cm germ cell tumour. After four cycles of chemotherapy with marked tumour regression (10 cm × 9.5 cm) and normalization of tumour markers, underwent a bilateral hybrid VATS with mobilization of the thymus, and its blood supply from the right side before making a small utility incision over the tumour and resecting en bloc with lingular segment of lung, phrenic nerve and pericardium. The pericardium was closed with equine pericardium and the left diaphragm was plicated through the same utility incision (Figures 10 and 11).

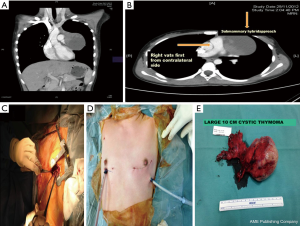
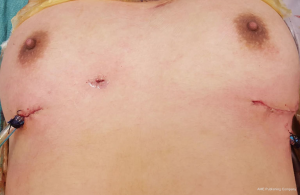
Between December 2012 and Dec 2016, 15 cases of selected anterior mediastinal thymic tumours were approached by bilateral VATS approach. Indications for surgery are shown in Table 1. Seven were females. Mean age 34.9 years (range, 14–74 years). Mean duration of surgery 148.9 minutes (range, 130–180 minutes). Mean length of stay was 3.5 days (range, 3–5 days).There were no mortality or morbidity. Mean size of tumour was 7.5 cm (range, 4–10 cm). Mean follow up is 24 months (range, 3–48 months). No local recurrences to date.
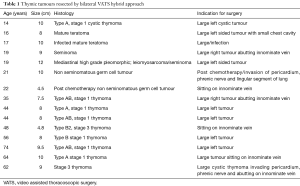
Full table
Preference card
- 5 mm 30 degrees camera lens;
- Two 5 mm Maryland dissectors;
- 5 mm endoscopic sucker;
- 5 mm endoscopic energy source (ultrasonic harmonic shears);
- 10 mm endoscopic retrieval bag.
Tips and pitfalls
- Gaining experience with VATS thymectomy should be gradual starting with non-thymomatous gland followed by small thymomas, larger tumours and finally invasive tumours.
- In myasthenia gravis completeness of thymectomy is crucial as it will affect long-term clinical outcomes. This is ensured at time of surgery by anatomical inspection of the resected gland for completeness and the thymic bed for perithymic fat and ectopic thymic tissue. Also always look for accessory horns especially under the innominate where it can be missed easily.
- Identification of both phrenic nerves should be routine and allows safe complete thymectomy to be performed. Thermal injury to the nerves can be minimized by using an energy source. Energy source also allows for quick dissection and safe coagulation of the thymic vessels
- Superior horns are usually dissected from the innominate vein first followed by the body and then the inferior horns of the thymus are sequentially mobilized. This is to prevent the weight of the gland from obstructing the surgical field of vision and making subsequent dissection from the innominate vein often difficult. This is especially important in thymomas where excessive manipulation should be avoided and the initially dissected horns can be used for traction when mobilizing the tumour last without risking injury to the tumour capsule.
- Though most surgeons use three ports, routine retraction through the additional 4th medial most 5 mm port has helped increase exposure and quicken the surgery especially in large hyperplastic glands with abundant perithymic fat.
- The semi supine position allows for quick conversion to a sternotomy if necessary.
- When both pleural cavities are opened one chest tube suffices as both pleural space functions as one compartment.
Conclusions
Surgeons attempting the VATS approach for thymic tumours should gain initial experience with VATS thymectomy for non thymomatous glands before carefully embarking on VATS approach for small followed by highly selected larger, invasive and complicated thymic tumours with a variety of VATS techniques. There should be no hesitation to covert to open technique if at any time during VATS the procedure is at risk of being oncologically compromised.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Vyas S, Agasthian T, Goh MH, et al. Thoracoscopic thymectomy in a previous sternotomy. Asian Cardiovasc Thorac Ann 2006;14:e108-10. [Crossref] [PubMed]
- Agasthian T. Clinical outcomes of VATS for thymomas. Interact Cardio Vasc Thorac Surgery Sept 2008; Suppl. 3 to 7:abstract 021. Available online: https://academic.oup.com/icvts/issue/7/Supplement_3
- Agasthian T, Lin SJ. Clinical outcome of video-assisted thymectomy for myasthenia gravis and thymoma. Asian Cardiovasc Thorac Ann 2010;18:234-9. [Crossref] [PubMed]
- Agasthian T. Can invasive thymomas be resected by video-assisted thoracoscopic surgery? Asian Cardiovasc Thorac Ann 2011;19:225-7. [Crossref] [PubMed]
- VATS Thymectomy. CTSNet, Thoracic Techniques. Oct 24, 2013. Available online: www.ctsnet.org
- Soon JL, Agasthian T. Harmonic scalpel in video-assisted thoracoscopic thymic resections. Asian Cardiovasc Thorac Ann 2008;16:366-9. [Crossref] [PubMed]
- Agasthian T. Right VATS thymomectomy. Asvide 2017;4:168. Available online: http://www.asvide.com/articles/1476
- Agasthian T. Bilateral VATS excision of WHO Type B2 Masaoka Stage 3 thymoma in a 35-year-old male with myasthenia gravis. Asvide 2017;4:169. Available online: http://www.asvide.com/articles/1477
- Agasthian T. Bilateral VATS hybrid for 10 cm cystic thymoma in a 14-year-old girl. Asvide 2017;4:170. Available online: http://www.asvide.com/articles/1478
Cite this article as: Agasthian T. Beyond the limits, extreme minimally invasive surgery in invasive thymic tumours. J Vis Surg 2017;3:58.