Robotics in general thoracic surgery procedures
Introduction
Minimally invasive surgery using tele robotic technology is not a novel idea anymore. When the da Vinci system was initially approved as the first robotic surgical system in 2000 by FDA, it was largely targeted at procedures requiring operating in very confined spaces and extreme dexterity such as cardiac surgery (1,2). However, the last two decades of twentieth century saw an exponential increase in utilization and acceptance of robotic technology in various surgical subspecialties. We now see the routine employment of robotic technology for a myriad of cases in thoracic, urology, general surgery, gynecology and colorectal procedures. The first use of robotic technology in general thoracic surgery was described by Melfi et al. from University of Pisa in Italy in 2002 (3). Since then, several larger series have been reported in the literature from various academic centers showing the safety of robotic thoracic surgery and comparable oncologic outcomes as compared to standard open and video-assisted thoracoscopic (VATS) approaches (4-7).
There are several reasons for growing interest and acceptance of robotic technology for thoracic surgery cases. Compared to open surgery, robotic technology provides a stable platform and is untiring. There is loss of haptic feedback but there is motion scaling and ability to operate with wristed instruments in very tight spaces thru small incisions (8). There is significant initial cost associated with the robot as compared to VATS but there are several technical advantages with the robotic approach. It provides 3D visualization with seven degrees of freedom. There is elimination of fulcrum effect with improved dexterity that makes microanastomosis possible in a minimally invasive fashion (8). These advantages are well recognized in thoracic surgical community. In this paper we will describe the utilization of 4 arms da Vinci Xi System for lung and mediastinal mass resection with discussion about patient selection, technique, common pitfalls and useful tips that we have learned from our experience at Memorial Sloan Kettering Cancer Center.
Components of the system and robotic set up
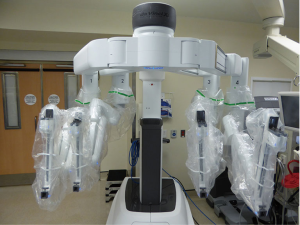
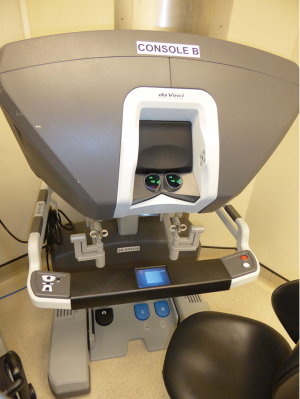
The da Vinci™ Xi Surgical System has three main components; the patient cart, surgeon console and the vision cart (Figure 1). The patient cart comprises the bedside console with the four surgical manipulator arms. In the latest Xi model, the endoscope can be attached to any of the four arms leaving three arms for instrumentation. All four arms including the endoscope are controlled from the surgeon console. This comprises the 3D image viewer, the master hand controls, and the footswitch panel to control electrocautery and allow switching between surgical arms and endoscope control (Figure 2). The endoscope has 30° angulation and a dual camera providing a binocular view of the surgical field to the surgeon console allowing 3D perception.
The vision cart contains a touch screen monitor to provide a view of the operative field to the bedside assistants and OR staff. An electrosurgical unit is integrated into the vision cart providing monopolar and bipolar energy to the various da Vinci™ Xi instruments mounted to the patient cart. The cautery is activated via the surgeon console. The robotic instruments and endoscope are interchangeable on the patient cart arms and are inserted into the patient through 8mm trocars.
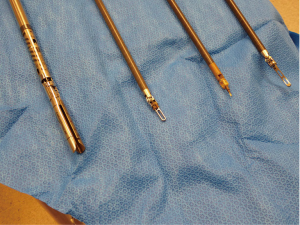
There are a variety of instrument options available (Figure 3). Typically for anatomic pulmonary resection, we use three instruments plus the robotic camera. For Right sided resections, these include a Spatula monopolar cautery for the right hand and a Fenestrated Bipolar grasper/dissector for the Left hand. The fourth robotic arm is used for tip up fenestrated grasper for lung resection independent of bedside assistant (Figure 4). An additional 5mm assistant port is sometime added more anteriorly between right hand and camera port for suctioning, additional retraction and passage of stapler as needed.
For anterior mediastinal/thymic work, patient is positioned supine with a shoulder role and a bump under the left chest to achieve a slight tilt towards the right side. Arms are then tucked on the sides which also allow proceeding with a median sternotomy if needed. We employ a 3 arms approach typically from the Left side. However, this can be tailored to right-sided approach if the mass is predominantly on the right side. Monopolar cautery is used in the right hand and fenestrated bipolar dissector/grasper in left hand. Dissection is typically started with a 0 degree camera but once the pleural on the right side is entered, we switch to a 30 degree robotic camera to make sure that all the thymic tissue on contralateral side is resected adequately and phrenic nerve is adequately identified and preserved. This approach has allowed us to avoid using a VATS camera from the contralateral side to visualize contralateral phrenic nerve.
Patient selection and workup
Patient selection for robotic lung resection essentially follows that the same principals as for traditional thoracoscopic surgery using VATS techniques. As with any surgical procedure, there is no substitute for sound judgment. Although for the novice robotic surgeon, ideal cases are early stage lung cancer and patients without induction treatment. However, with experience, more advance cases such as IIIA and even induction cases can be safely performed using the robotic technology.
Absolute contraindications:
- Vascular invasion requiring venous or arterial reconstruction;
- Superior sulcus/Pancoast tumors.
Relative contraindications:
- Induction Chemo/RT;
- Chest wall invasion;
- Redo thoracoscopy;
- Diaphragmatic/Pericardial involvement requiring resection and repair;
- Bronchial sleeve resections.
Pleural adhesions have been cited as a relative contraindication for a VATS approach. This is less so for robotic approach in our experience as the visualization and the extra dexterity makes extensive adhesiolysis technically more feasible compared to traditional VATS approach.
Pre-operative preparation
Preoperative work up is obtained in all patients based on current NCCN guidelines. All patients undergo initial CT chest with intravenous (IV) contrast and PET/CT for clinical diagnosis and staging. Image guided tissue diagnosis is obtained as dictated by tumor location. Pulmonary function tests (PFTs) are obtained in all patients. MRI of the brain is obtained in stage II and above. Quantitative ventilation/ perfusion scanning are obtained in patients with borderline PFTs. Additional testing is obtained for surgical clearance as dictated by individual patient’s comorbidity status.
Equipment preference card for robotic assisted segmentectomy/Lobectomy/Lymph node dissection
Equipment:
- Olympus mattress;
- Bair hugger with lower body blanket set @ 43 °C;
- SCD machine & boots;
- da Vinci robot—set up robot on the same side as the affected side, set up all 4 arms.
Key consideration:
- Will inject 5% Marcaine or Exparel into port sites in the beginning of the case and intercostal blocks at the conclusion of the procedure;
- If the patient has an epidural local will not be used.
Position:
- Patient is in lateral position, affected side up. Two pillows between legs, ether screen, arm foam sling, Abd pads, Silk tape.
Prep:
- Patient prepped from shoulder to hip, bedside to bedside, with chloraprep.
Drape:
- Four “blue sticky” drape towels to square incision;
- Endoscopy drape.
Instruments:
- Thoracic da Vinci Xi instrument tray;
- da Vinci Xi accessory tray;
- da Vinci Xi 30 degree scope 8 mm;
- MIS VATS tray;
- Endoduval ×2;
- Mediastinal needle;
- I drive stapling device;
- (MIS) 5 mm suction tip;
- THOR snowden clamp 5 mm × 32 cm;
- Thoracic retractor tray;
- Thoracic dissecting tray;
Sutures:
- 2–0 Vicryl CT-1 ×1 for utility incision muscle closure;
- 2–0 Vicryl UR ×1 for port muscle closure;
- 4–0 Monocryl FS-1 ×2 for subcuticular closure;
- 0 silk FSL for chest tube site.
Standby (if procedure converts to “open”):
- 0, 2–0, 3–0 silk strands;
- 3–0 PDS SH CR for suture ligature;
- 3–0 silk SH CR for suture ligature;
- #2 Vicryl for pericostal closure;
- 0 Vicryl CT ×4 for muscle & fascial closure;
- 2–-0 Vicryl CT ×2 for subcutaneous closure;
- 0 silk FSL ×2 for chest tubes.
Ports:
- 8 mm Xi ports ×4;
- 5 mm applied medical trocar—standby;
- Xi port caps for CO2 insufflation—standby;
Supplies:
- Xi instrument drape ×4;
- Xi column drape;
- Foley catheterization kit;
- 28 straight chest tube;
- Cherries;
- Anchor bags—have all sizes available;
- Endopeanut (available);
- Surgicel;
- Arista;
- 25 G hypo 1½ inch;
- Progel with long tip applicator;
- mall laps ×2;
- Pulse-wave tubing;
- Plastic Yankauer;
- Dermabond;
- ***Insufflate selectively***—please do not open trocar seals.
Staples:
- I drive ultra powered stapler device;
- Endo GIA DLU—purple and tan curved tip and regular;
Postoperative considerations:
- Chest tube on water seal secured with chest tube ties.
Procedure
All patients have a type and screen checked on the day of surgery on admission. We do not routinely place epidural analgesia catheters in patients undergoing robotic lung resection. This has been replaced with intraoperative intercostal nerve block with either standard 50% Marcaine solution or long acting liposomal bupivacaine solution (Exparel, Pacira Pharmaceuticals, Inc.). This is augmented with postoperative analgesia with IV patient controlled analgesia (PCA) and non-narcotic analgesics. This strategy allows for less hemodynamic concerns post operatively, early ambulation, lower incidence of urinary retention and faster progression to per oral pain control in our patients.
In the operating room, patient is initially placed in supine position and flexible bronchoscopy is performed if not done previously. Standard antibiotic prophylaxis is given within one hour of incision per NSQIP guidelines and DVT prophylaxis instituted with sequential compression device on lower extremities and 5,000 units of sub cutaneous heparin on all patients. Pt is then intubated with double lumen endotracheal tube with isolation of the affected side. Patient is then positioned in lateral decubitus position with surgical side up. All the pressure points are appropriately padded. Table is then flexed to accentuate rib spaces.
Port placement and robot docking
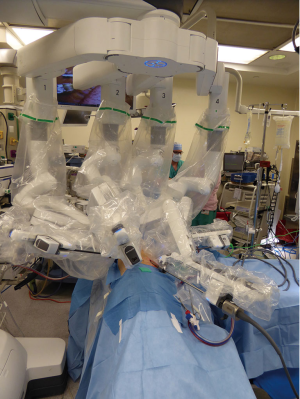
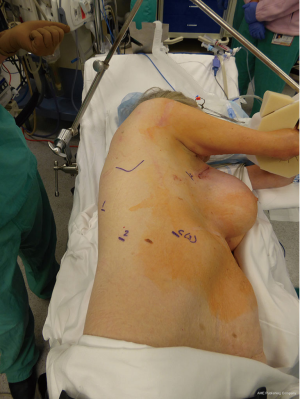
For lung resections, patient is placed in lateral decubitus position as is standard for open or VATS lung resections (Figure 5). The da Vinci Xi robotic platform is positioned on the affected side. All four arms are draped sterilely. The Xi system is more forgiving in terms of positioning of patient-side cart in relation to the operating room table as compared to the former Si system. In general, the patient-side cart is brought in from posteriorly at about 90 degrees to the spine. For the Xi system, all ports are 8mm which makes it more versatile and allows to switch robotic camera between different ports for optimal visualizations during various portions of the procedure. Camera port is usually placed first in 8–9 interspace in posterior axillary line. Robotic camera is then used to target the major area of dissection at either the lung hilum or the major fissure based on the lobe or segment in question. The remaining arms are then auto configured by the onboard computer in relation to the camera port. We place a 12 mm port in 8th interspace in the line below the tip of Scapula, for bipolar robotic dissecting forceps. This port is also used for later introduction of I drive ultra powered stapler device (© 2016 Medtronic, Minneapolis, MN, USA). An 8 mm posterior port is placed in 5th–6th interspace posterior to the tip of scapula for introduction of tip up fenestrated grasper for lung retraction. We routinely make a 2 to 2.5 cm non rib spreading utility incision in 4–5th interspace anteriorly in the posterior axillary line over the hilum. This port is used to introduce the monopolar spatula for energy dissection. Although a total port robotic approach has been described and successfully performed by other groups (9), we feel that an incision in this location provides several advantages. In particular for the novice robotic surgeon, this port allows for rapid hemostatic control in the event that a pulmonary arterial or other vascular injury was to occur. This is crucial from a patient safety perspective while adopting a new technology in the operating room. In author’s experience, one of the ports eventually has to be widened to this size for specimen extraction at the end of the case even with the total port approach. We make this at the widest portion of the ribs more anteriorly where this can also be hidden under the infra mammary fold for better cosmesis.
Lymph node dissection
At our institution, we emphasize on a complete thoracic lymph node dissection for all lung cancer cases. Dissection is usually started with taking down the inferior pulmonary ligament with removal of level 8 and 9 lymph nodes. We prefer to perform the posterior dissection first. Emphasis again is on complete level 7 and 10 lymph node dissection. This is followed by anterior hilar dissection with further lymph node harvest. This facilitates vascular dissection for later stapling depending on the anatomic lobe in question (Figures 6,7).


Hilar dissection
The key issue to keep in mind is the lack of tactile sensation for the operating surgeon. This places more emphasis on real time visual feedback for dissecting hilar structures which can be a major adjustment for the novice robotic surgeon no matter how experienced they are. It also becomes more important to pay particular attention to not cause inadvertent injuries outside of surgeon’s field of vision. The da Vinci Xi surgical platform has additional protective features against such injuries using the guided tool exchange for precise instrument exchanges close to hilum.
Venous circulation is usually isolated first after confirming adequate venous drainage of remaining lung. More recently, we now prefer to use I driveTM ultra powered stapler device (© Medtronic, Minneapolis, MN, USA) for stapled division of hilar vessels and airway for several reasons. The automated feature allows for a stable stapling platform and eliminates excessive undesirable hand movement which is useful for new trainees and physician assistants (PA). It is also reusable up to 25 staple loads and minimizes cost as compared to robotic stapler. We have also found that the use of straight jawed stapler allows for more controlled passage around the vessels. However, it is completely reasonable to use the traditional non-motorized or the robotic stapler for these steps depending upon individual surgeons comfort level and we have used various stapling devices at times (Figure 8).

After venous control, arterial supply is divided as appropriate and bronchus is usually divided at the end. There are variations to these steps for certain lobes, the details of which are beyond the scope of this publication but as an example in case of the right middle lobe, after dividing the middle lobe vein; it is usually technically more efficient to divide the middle lobe bronchus prior to dividing the pulmonary arterial branches. Fissure is then completed with multiple fires of medium thickness linear cutting stapler. Specimen is extracted in a bag thru the anterior utility port and examined on the back table for adequacy of gross margin.
Role of team members
We perform most lung and thymic resections with an attending surgeon, a thoracic fellow and a MIS trained PA. The attending surgeon operates from the master console after the bedside robotic platform is adequately docked and instruments mounted on the robotic arms. The thoracic fellow also scrubs out at this point and manages the slave/ teaching robotic console. He/She is then proctored to perform appropriate steps during each case such as taking down the inferior pulmonary ligament or lymph node dissection giving increasing operative independence based on individual’s experience and skills. The PA is the primary bedside assist. From the very beginning of thoracoscopic surgery at our institution, we believe in training our operative PAs thru variety of simple and complex operative steps. As such, on routine VATS cases, PAs are allowed to fire staples loads on lung parenchyma and with increasing experience, also across vascular structures and bronchi. This has helped us tremendously in relying solely on our mid-levels as the primary bedside surgeon and obviating the need of having another thoracic surgeon in the room.
Post-operative management
We tend not to get epidural catheters for our robotic cases any more in attempts for early postoperative ambulation. Standard IV PCA is used along with scheduled IV Toradol every 6 hours. We do not send our patients to ICU post op and refrain from keeping them in recovery room overnight unless there is concern for postoperative bleeding, prior respiratory insufficiency or other significant co-morbidities. We remove chest tubes mostly on post op day 1 when the output is serosanguineous and less than 10 cc/kg for 12–24 hours. We do not routinely get chest X-rays post chest tube removal.
Robotic thymectomy/Anterior mediastinal mass resection
Preoperative neurology evaluation is critical for patients with myasthenia gravis. Disease severity is determined preoperatively and patient is appropriately prepared for surgery using standard therapy vs. IVIG or plasmapheresis. Depolarizing agents are avoided in the operating room for these cases. For robotic thymectomy and midline anterior mediastinal mass resection, we typically employ a left sided approach although this is tailored to right side approach for predominantly right sided anterior mediastinal masses. We place the camera port first typically in 4th–5th intercostal space in anterior axillary line. The left arm is placed in the inframammary fold in mid-clavicular line and a bipolar fenestrated grasper is used through this arm. The right arm is placed thru 2rd−3rd intercostal space in anterior axillary line and a monopolar spatula cautery is used thru this arm. We also use robotic harmonic scalpel in lieu of monopolar spatula cautery for ligation of small vessels and superior dissection of thymic horns.
We routinely use CO2 insufflation for these cases, which also helps with dissection in mediastinal plains (Figure 9).

There are several published series of robotic thymic resection in the literature. The initial experience was focused on myasthenia gravis but there is increasing studies focusing on the robotics in the primary treatment of thymoma. Ruckert et al. performed a retrospective review of minimally invasive thymectomies performed for myasthenia gravis and compared the traditional VATS approach to robotic approach in 153 consecutive patients (14). There were no differences in the age and disease severity between the two groups. At 42 months, they found a cumulative complete remission rate of myasthenia gravis for robotic cases at 39.25% vs. the non-robotic thymectomy patients which was 20.3% (P=0.01) (10). The largest series of robotic thymectomy for thymoma is from Marulli et al. which is a multi-institutional European experience. They reported 134 patients undergoing robotic thymectomy for thymoma. Thirty-eight percent were approached from left side; 59.8% from right side and 2.2% underwent bilateral approach. Fifty-two percent were associated with myasthenia gravis. They reported a 97% 5-year survival rate (15).
Tips, tricks and pitfalls
Robotic time out
We routinely perform a robotic time out after the standard surgical time out at our institution. The purpose of robotic time out is to have all the team members in addition to the surgical team, including anesthesia technicians/residents, circulating staff and operating room nurses know exactly what to do in case of an emergency or inadvertent vascular injuries. Since undocking the robot and achieving manual control of any injury may unwillingly take some time, especially when the attending surgeon is not scrubbed on the field, our usual strategy is to apply gentle pressure with the lung compressed over the hilum using the robotic grasper or with a gauze sponge thru the utility incision as one would with an open case. This allows for stable gentle pressure and adequate temporary control of bleeding. The staff is instructed to refrain from emergently undocking the robot unless instructed by staff surgeon because it might be utilized for temporary hemostasis. A rush to undock the robot can also cause even more severe injuries while the attending surgeon is trying to provide temporary means of hemostasis using the robotic arms.
In case of emergency, the instructions for anesthesia team are to have two units of blood available in the room as quickly as possible and support the patient with IV fluids and pressor medications as necessary. The instructions for the circulating nurse are to contact another senior thoracic surgeon immediately while staying available in the operating room to grab supplies as necessary. This back up attending surgeon is also identified and confirmed to be available in the building as part of robotic time out at the beginning of the case.
Reliance on a second thoracic surgeon as bedside assist
This is a common error that is often overlooked. At our institution, we initially performed robotic cases with two attending surgeons and a PA in the room. However, as previously explained, we were quickly able to transition our PA as the sole bedside assistant. Relying on a second thoracic surgeon can be challenging and can potentially be a hurdle is setting up a busy robotic practice due to scheduling conflicts. This also takes away valuable surgical experience for the trainees by having another surgeon in the room.
Failure to incorporate residents via teaching console
As mentioned previously, we routinely have our fellows scrub out with the attending surgeon and perform individual operative steps under direct supervision. At the beginning of thoracic surgical rotation, the fellows are expected to complete online training modules that go over basic robotic set up, port placement and instrumentation. After successful completion of this, they also undergo a dry lab with Intuitive Inc representative to understand the docking, instrument placement and arm articulation. They are then expected to spend certain hours completing training modules on virtual simulator before that are allowed to perform steps during live surgeries. We consider progressing residents in this graduated fashion as the safest and most efficient way of introducing them to these very complicated minimally invasive operations.
Failure to monitor outcomes
We encourage new surgeons in this field to have a systematic way of monitoring outcomes of robotic thoracic cases at their home institutions. This can be challenging depending on available resources, however, certain variables can be easily monitored using basic data from these cases. Most essential element for outcomes surveillance is making sure that the basic oncologic principles of lung cancer surgery are maintained consistent with Cancer and Leukemia Group B (CALGB) consensus and there is no compromise in disease eradication and pathologic staging parameters such as adequate surgical margin and lymph node harvest. As we will discuss later in this paper, robotic technology has been showed in several studies to provide same long term oncologic results as compared to open surgery (16). Additional outcome parameter such as analgesic pain requirements, early ambulation, postoperative complications such as pneumonia, DVT, hospital length of stay and cost considerations are also valuable quality control measures to keep track of.
Outcomes for robotic lung resections
The first published series of robotic assisted lung resection was reported by Melfi et al. in 2002 (3). This heterogenous group of robotic thoracoscopic procedures included five lobectomies and demonstrated the feasibility of using the da Vinci™ system in thoracic surgery with no operative mishaps and appropriate functioning of the robotic arms for the procedure.
Following this landmark paper, numerous reports of experience using the robotic system for lung resection followed including our own experience from Memorial Sloan Kettering Cancer Center in New York (4). Our series consisted of 34 patients having robotic assisted, minimally invasive lobectomy with four conversions to thoracotomy (12%). All types of lobectomy were performed showing the versatility of the robotic system. No perioperative deaths were observed and a 26% morbidity rate, comparable to techniques of open and VATS lobectomy. Chest tube duration (3 days) and length of hospital stay (4.5 days) were comparable to standard techniques. All patients underwent an R0 resection and had a median of 4 lymph node stations dissected. Our results have been replicated by numerous institutions around the world in the subsequent years (7,17-19). These studies report perioperative mortality rates from 0–3%, morbidity rates from 10–26%, conversion rates of 0–12% and median length of stay of 2–6 days.
The current robotic surgical platform affords more intuitive movements, greater flexibility and high definition three dimensional vision to overcome the limitations of traditional thoracoscopy and will result in wider adoption of this technology by the thoracic surgeons (20). In a multi-institutional retrospective review of patients undergoing robotic lobectomy for early stage NSCLC, the long term 5 years stage specific survival was consistent with prior results with VATS and open thoracotomy (21). Additionally, robotic approach is particularly helpful in performing a complete lymph node dissection and lymph node yield is higher compared to VATS approach while providing the benefits of minimally invasive surgery with shorter hospital stay and improved pain control (16). A common criticism against robotic technology is the tremendous initial cost and ongoing expenditures with disposable robotic instruments and annual service fees. We have looked at the relative total costs of pulmonary resections incurred using thoracotomy vs. VATS vs. robotic. We have shown that the actual cost associated with a minimally invasive VATS approach to lobectomy is substantially lower than that resulting from a thoracotomy. The bulk of the improvement is the direct result of a decreased length of hospital stay afforded by use of VATS and Robotics (22). The increase in robotic cost is secondary to initial cost and specialized non reusable robotic instruments. However, the annual amortized cost with the robotic approach decreases with increasing number of robotic cases per year at any institution (22).
Conclusions
Reported experience in the literature shows robotic pulmonary and anterior mediastinal mass/thymic resections to be safe and reproducible techniques. It provides a technically safe and oncologically sound alternative to conventional open and thoracoscopic VATS lobectomy. Randomized trials comparing the technique to standard open approach and conventional VATS surgery are not available and are unlikely ever to be conducted. Papers from institutions across the world, however, report equivalent perioperative outcomes. Long term oncologic efficacy has also been demonstrated with evidence of superior nodal dissection and therefore accurate staging in lung cancer patients.
This intuitive technique should remove the technical barriers that prevent some surgeons from adopting a minimally invasive approach. Fears over excessive costs have been allayed compared to standard thoracotomy. It is anticipated that as the technology becomes more widely adopted, associated costs should fall promoting yet wider adoption across the thoracic surgical community.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Robotic device cleared for heart surgery. FDA Consum 2004;38:6. [PubMed]
- Zenati MA. Robotic heart surgery. Cardiol Rev 2001;9:287-94. [Crossref] [PubMed]
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Gharagozloo F, Margolis M, Tempesta B. Robot-assisted thoracoscopic lobectomy for early-stage lung cancer. Ann Thorac Surg 2008;85:1880-5; discussion 5-6.
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Lanfranco AR, Castellanos AE, Desai JP, et al. Robotic surgery: a current perspective. Ann Surg 2004;239:14-21. [Crossref] [PubMed]
- Cerfolio RJ. Total port approach for robotic lobectomy. Thorac Surg Clin. 2014;24:151-6. v. [Crossref] [PubMed]
- Latif MJ, Park BJ. Left upper lobe hilar dissection—part 1. Asvide 2017;4:126. Available online: http://www.asvide.com/articles/1434
- Latif MJ, Park BJ. Left upper lobe hilar dissection—part 2. Asvide 2017;4:127. Available online: http://www.asvide.com/articles/1435
- Latif MJ, Park BJ. Use of robotic stapler for division of RUL hilar vessels. Asvide 2017;4:128. Available online: http://www.asvide.com/articles/1436
- Latif MJ, Park BJ. Robotic assisted resection of locally advanced thymoma. Asvide 2017;4:129. Available online: http://www.asvide.com/articles/1437
- Ruckert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [Crossref] [PubMed]
- Marulli G, Maessen J, Melfi F, et al. Multi-institutional European experience of robotic thymectomy for thymoma. Ann Cardiothorac Surg 2016;5:18-25. [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term Survival Based on the Surgical Approach to Lobectomy For Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2017;265:431-7. [Crossref] [PubMed]
- Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg 2011;23:36-42. [Crossref] [PubMed]
- Gharagozloo F, Margolis M, Tempesta B, et al. Robot-assisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg 2009;88:380-4. [Crossref] [PubMed]
- Jang HJ, Lee HS, Park SY, et al. Comparison of the early robot-assisted lobectomy experience to video-assisted thoracic surgery lobectomy for lung cancer: a single-institution case series matching study. Innovations (Phila) 2011;6:305-10. [Crossref] [PubMed]
- Veronesi G. Robotic thoracic surgery: technical considerations and learning curve for pulmonary resection. Thorac Surg Clin 2014;24:135-41. v. [Crossref] [PubMed]
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [Crossref] [PubMed]
- Park BJ, Flores RM. Cost comparison of robotic, video-assisted thoracic surgery and thoracotomy approaches to pulmonary lobectomy. Thorac Surg Clin 2008;18:297-300. vii. [Crossref] [PubMed]
Cite this article as: Latif MJ, Park BJ. Robotics in general thoracic surgery procedures. J Vis Surg 2017;3:44.