Video-assisted thoracoscopic surgery and open chest surgery in esophageal cancer treatment: present and future
Introduction
Franz Torek performed the first successful transthoracic esophagectomy with reconstruction by an external rubber tube in 1913 (1). In the following decennia the basic oncologic principles of esophagectomy and techniques of reconstruction were worked out. Roughly since the sixties of the previous century, esophagectomy became the mainstay of the treatment with curative option for esophageal cancer patients, not in the least thanks to the development of better anesthetic and intubation techniques and better analgesia. Perioperative mortality, once higher than 70% in the 1930’s (2) dropped dramatically to 2% or even lower in highly specialized centers nowadays. Nevertheless, perioperative morbidity is still high up to 50% or more (3). Although 5-year survival after esophagectomy occasionally reached 35% to 40% in some centers, for a long period the long term survival remained dismal and almost didn’t improve anymore for decennia (4).
In order to reduce perioperative morbidity and to improve 5-year survival, minimally invasive techniques, as well as multimodality treatment have been introduced in esophageal cancer treatment mainly since the beginning of this millennium (5-8). As a result perioperative morbidity and especially pulmonary complications could further be reduced (9) and long term survival further improved (10) as shown in the most recent randomized trials. This opened the window for an increasing interest for evaluation and comparison of quality of life obtained by different treatment modalities in today’s esophageal cancer patients.
At the author’s institution, these technical innovations and the multidisciplinary (r)evolutions in esophageal surgery were first critically reviewed, then introduced and further evaluated thereafter always keeping in mind the greatest respect for the basic surgical and oncological principles as well as the well-being of the patient. Tri-modality treatment for esophageal cancer in separate teams evolved into a multidisciplinary team approach within an individual therapeutic plan tailored for every single patient presenting with a cancer of the esophagus or gastro esophageal junction (GEJ).
The first totally minimally invasive esophagectomy (MIE) in Leuven was done in 2003 in a patient with an early (T1aN0) stage cancer that today would have been treated by endoscopic mucosal resection (EMR). The procedure was a three-stage procedure performed through a right sided VATS in left lateral decubitus followed by a totally laparoscopic procedure and left cervicotomy in supine position. After a single surgeon learning curve in over 100 patients (11) with special attention for not compromising the oncologic principle as applied in open surgery (in particular the lymph node dissection), more advanced esophageal cancers became accepted for this type of surgery, also including resections after neoadjuvant chemoradiation therapy. Together with this learning curve, mean number of resected lymph nodes became even higher than in open surgery (12). Other surgeons of the team subsequently became familiarized with MIE as well. Based on several literature reports and personal on-site visits of expert centers, the next step was to perform the right VATS procedure in prone position, which facilitated visualization, lymph node dissection and teaching possibilities (13-15), but challenged the anesthesiological team to keep airway control in any situation.
Therefore a standard protocol was written including practical guidelines allowing surgeons, anesthesiologists and operating theater nurses to familiarize themselves with this technique. In this article minimally invasive esophageal cancer treatment will be discussed as part of a multidisciplinary treatment in our department.
Principles of surgical treatment
It is generally accepted that surgical resection should only be performed with curative intent. Resection is ill-advised when macroscopically incomplete resection (due to invasion of adjacent structures and/or non-resectable metastases) is to be expected. Absolute contra-indications for esophagectomy include local tumor invasion of non-resectable neighbouring structures (T4), carcinomatosis peritonei or thoracalis, hematogenous parenchymatous metastases involving e.g., the liver and non-resectable metastatic lymph nodes.
The pattern of lymphatic dissemination is difficult to predict, but carcinomas of the proximal and middle thirds of the esophagus preferably metastasise to the cervical region, whereas more distally situated tumors and tumors of the gastro-esophageal junction more commonly metastasise to the lymph nodes around the celiac artery.
Resectable metastatic lymph nodes in the region of the primary tumor, including the celiac trunk and its trifurcation for distal third tumors and cervical nodes for middle and proximal tumors, are not necessarily a contra-indication for surgery. The presence of lymph node metastases, however, has a negative influence on survival, even following extensive lymphadenectomy.
The early-stage lymphatic dissemination as well as completeness of tumoral resection (R0) pose challenges for radical surgical treatment and are still a matter of debate. The concept of extensive en bloc resections was already reported in 1963 (16), but its associated mortality of more than 20% in the original report, discouraged general acceptance. Skinner (17) and Akiyama et al. (18) reintroduced the concept of en bloc resection combined with extensive lymphadenectomy. Ultimately, they were able to reduce operative mortality to 5%, with 5-year survival rates of 18% and 42% respectively. These numbers are opposed to these after transhiatal esophagectomy introduced in the western world by Orringer et al. in 1978 (19). The rationale here is that the surgical intervention will not be able to influence the natural course of the disease. Therefore the intervention is merely restricted to the esophagectomy without making efforts to perform more extensive lymphadenectomies.
The radical en bloc resection, as opposed to the standard resection, aims at performing an as wide as possible peritumoral with an en bloc lymph-node resection of the middle and distal thirds of the posterior mediastinum.
The two-field lymph node dissection incorporates, besides a wide local excision of the primary tumor, a lymphadenectomy of the entire posterior mediastinum, also including the subcarinal nodes and up to the nodes along the left recurrent nerve and the brachiocephalic trunk. In the abdomen it includes the lymph nodes along the celiac trunk, common hepatic and splenic arteries, as well as the lymph nodes along the lesser gastric curvature and in the lesser omentum.
There is an increasing consensus that the optimum of resected lymph nodes should at least be 23 nodes (20).
The three-field lymph node dissection. The pattern of lymphatic dissemination is not restricted to the thorax and abdomen. About 20% of the patients with a distal tumor present with metastasis in the cervical region (21). In this operation, besides the already mentioned removal of thoracic and abdominal nodes, the cervical field is defined as the third field and includes the paraesophageal nodes and the nodes lateral to the carotid vessels as well as the supraclavicular nodes. Three field lymphadenectomy is mainly practiced in the far East and is recommended in squamous cell carcinoma of the middle and upper third of the esophagus.
These considerations on radicality of resection and extent of lymphadenectomy are the rationale to justify a transthoracic approach as opposed to the transhiatal approach for which the rationale is merely based on an effort to decrease perioperative morbidity and possibly postoperative mortality.
Recent observations indicate that the results of radical esophagectomy are much better than commonly quoted and today with a proper selection of patients overall 5-year survival figures reaching 40% are frequently reported.
Patient selection and workup
All patients (minimally invasive or open surgery) undergo an oncological workup including flexible endoscopy with biopsy of the tumor, endoscopic ultrasonography of the tumor and reachable lymph nodes (in case there is no stenosis) and full body PET-CT with intravenous contrast. For early tumours, an endomucosal resection (EMR) can be performed as a staging procedure (distinguishing T1a fromT1b) becoming subsequently a definitive treatment in case of T1a lesion, unless lymphatic invasion and /or poor differentiation. A bronchoscopy can be performed in selected cases to rule out T4 stage or second primary cancer in the lung especially in case of squamous cell carcinoma.
Over the recent years neoadjuvant therapy has become the standard in locally advanced cancer of the esophagus and GEJ accounting for about two thirds of all patients to be treated with curative intent.
Medical operability assessment also includes a functional assessment including electrocardiogram, pulmonary function tests and cardiac stress testing. Echo-Doppler of the carotids will be performed in vascular high risk patients. Any other co-morbidity will be thoroughly evaluated and treated lege artis when needed. The medical operability will be tested not only at the start of neoadjuvant therapy but both oncologic and medical operability will be reassessed again afterwards before the surgery in order to evaluate the impact of the neoadjuvant therapy.
Open versus MIE: indications
Our center is a high volume tertiary referral center performing approximately 120 esophagectomies for cancer per year.
Patients with resectable distal esophageal or GEJ tumors and small mid-thoracic tumors are usually operated on by totally minimally invasive surgery, being VATS and laparoscopy, in general a McKeown procedure. Patients with bigger mid-esophageal tumors, especially patients with squamous cell carcinomas after neoadjuvant therapy, are usually operated on in a hybrid fashion by classic right thoracotomy, followed by laparoscopy and left cervicotomy. Patients over 80 years or more fragile patients suffering from multiple co-morbidities (approximately 15% of cases) with distal or GEJ tumors are preferably operated on by open left thoraco-abdominal approach, as it is faster (shorter narcosis time) and in most of these patients an intrathoracic anastomosis can be performed (lower risk of anastomotic leakage). Other indications for open surgery are patients presenting with subcardia tumors extending downwards on the fundus and who will require total gastrectomy (approximately 15% of cases) or patients who have had major upper GI surgery e.g., multiple redo antireflux surgery, bariatric surgery…..
Finally the choice of open versus MIE is left to the discretion of the surgeons. As not all surgeons have stepped in into the prone MIE resections notwithstanding a still growing trend to adopt the MIE in prone position.
All together this resulted in a 47% prone MIE in 2016.
Pre-operative preparation
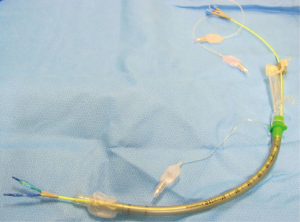
Patients are prepared for surgery with an epidural anaesthetic catheter, placed at the level of the 7th thoracic intervertebral space under local anaesthetic. Afterwards, the patient is positioned in supine position to prepare for general anaesthesia. An arterial line is brought in a radial artery. After induction with propofol and starting the general anaesthesia with sevoflurane, a nasogastric tube is inserted and the patient is intubated using a single lumen reinforced endotracheal tube. An endo-bronchial blocker in then positioned under bronchoscopic evaluation. The use of a blocker with two balloons (each of them positioned in one bronchus) (Figure 1) helps stabilizing the blocker in a correct position.
Next, a central line is inserted preferably in the right subclavian vein. This allows the surgical team to drape the neck for the left cervicotomy but even so for a possible bilateral cervicotomy if a three-field lymphadenectomy is indicated. It also facilitates the postoperative care for the central line compared to a jugular central line in preventing central line infections. Finally a urinary catheter is inserted to monitor urinary output during and after the intervention and sequential pneumatic compression devices are put around the legs during the whole procedure as prophylactic treatment for thrombo-embolic phenomenons.
Positioning and equipment preference card
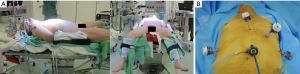
First stage: the patient is positioned in prone position for thoracic esophageal dissection and lymphadenectomy. Installation in prone decubitus position requires a standard device in order to support on the head, chest and pelvis. Pillows are placed to support shoulders and pelvis leaving the abdomen free for breathing excursions. The head is placed in a special support with integrated mirror allowing the anesthesiologist, even in prone position, to evaluate the face of the patient and the position of the tube (Figure 2A). Positioning of the right arm is very important in order to get abduction of the scapula. The arms are positioned on a support device in flexion of the shoulder and elbow (Figure 2B). In this way the area between the spine and the inner edge of the scapula is widened.
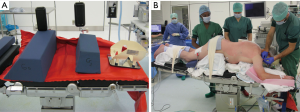
The patient is further stabilized on the table using a bean-bag and two side supports on the left side of the chest (Figure 2A). This allows, if necessary, rotation of the table up to 45° causing the patient to slide alongside those supports in a more lateral decubitus position, in that way making an urgent thoracotomy easier.
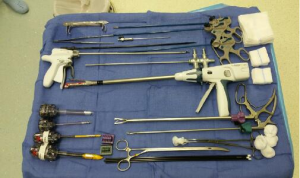
Equipment for patient installation and surgical equipment are described in card 1 in supplementary.
Second and third stage: the patient is positioned in supine position with neck in hyperextension and the face turned to the right for laparoscopic mobilization of the stomach, gastric tubulation and lymphadenectomy combined with the anastomosis via a left cervicotomy.
The legs are placed in the leg support without flexion in the hips in order to have maximal range of motion with the laparoscopic instruments. The neck is already placed in hyperextension and somewhat to the right to be ready for stage 3 .The abdomen and neck are prepped and draped together.
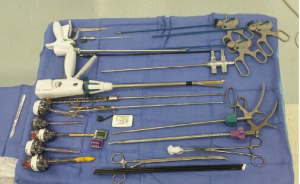
Equipment for patient installation and surgical equipment are described in cards 2 and 3 in supplementary.
Procedure
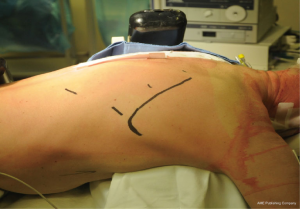
First stage: the surgeon stands on the right side of the patient with the first assistant on his or her right side, both looking to the monitor in front of each of them (Figure 4). The right hemithorax is prepped and draped, making sure that enough lateral skin is free to perform an urgent thoracotomy when needed.
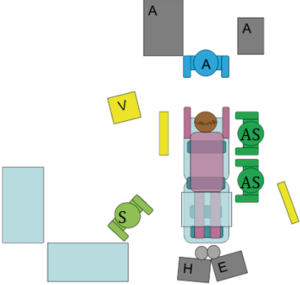
Four trocars are placed along the inner edge of the right scapula: the first at the level of the tip of the scapula for the thoracoscope (usually a 10 mm 30°), the second at the level of the 4th intercostal space (5 mm), the third at the level of the 8th intercostal space (12 mm) and the last one (5 mm) at the level of the 2nd intercostal space, as working trocar for the assistant (suction, retraction) (Figure 5). After introduction of the first trocar, insufflation with carbon dioxide with positive pressure of 5 to 8 mmHg is initiated in order to compress the right lung allowing for an adequate visualization of the posterior mediastinum.
Typically, the dissection is started with the transection of the inferior pulmonary ligament. The dissection is extended by incising the mediastinal pleura at the level of the pericardium and alongside the inferior pulmonary vein up to the right main stem bronchus. The lower edge of the bronchus is visualized by dissecting the right lower part of the subcarinal lymph nodes adjacent to the right main stem bronchus. This package of nodes is subsequently dissected away from the pericardium, staying en bloc and connected with the esophagus (Figure 6). The pleura overlying the right main stem bronchus is incised up to the crossing azygos vein. This arch of the azygos vein is completely dissected and transected using vascular endostaplers (2 mm stapler height).The underlying intercosto-bronchial artery is clipped and transected as well (Figure 7). The esophagus with the concomitant right vagus nerve is pushed upwards and the connective tissue between esophagus and membranous part of the trachea is carefully divided pushing the esophagus away from the trachea. This dissection is continued to the left border of the trachea until the left paratracheal gutter is entered (Figure 8). Now, very importantly, careful dissection will expose the left recurrent nerve that has to be further dissected out without using any electro cautery (Figure 9). Having created a window behind the esophagus, the esophagus is pulled downwards. From above the esophagus a section plane between this structure and the beginning of the aortic arch is made starting from the distal border of that earlier created window. This will bring into view the posterior wall of the left main stem bronchus which is carefully liberated exposing in that way the carina and the right trachea-bronchial corner and the left border of the subcarinal lymph node package. This lymphatic tissue is dissected off the main stem bronchus and thus completing the en bloc removal of the subcarinal lymph nodes in one piece (Figure 10). Now the dissection is continued flush along the descending aorta taking the thoracic duct en bloc with the esophagus. The thoracic duct is clipped and transected several centimeters below the tumor, above the diaphragm. Also at the opposite side, the dissection is performed away from the esophagus flush along the pericardium taking all lymph nodes and this until the diaphragmatic pillars are reached (Figure 11) Attention is now moved towards the superior mediastinum. After incising the mediastinal pleura left from the superior caval vein, the fatty tissue and lymph nodes between the vein and the left anterolateral part of the trachea are dissected and removed (Figure 12). This is followed by the lymph node dissection along the right recurrent nerve. Pulling on the remaining proximal part of the right vagal nerve facilitates the visualization of and dissection along the right recurrent nerve that is turning around the right subclavian artery. All small vascular branches are clipped and transected as no electrocoagulation is allowed here (Figure 13). Next comes the dissection along the already in part dissected left recurrent nerve removing all adjacent lymph node up well in to the base of the neck. Again electro cautery in the neighborhood of the recurrent nerve is avoided (Figure 14).









Figure 15 shows nicely the end result i.e., the thorough lymphadenectomy and en bloc dissection of the entire posterior mediastinum.

When this has been achieved, careful hemostasis is performed and classically two chest drains are placed using therefore the two most basal ports. One classic 28 F chest drain apical and one flexible 19 F silicon drain on the diaphragm. The right lung is reinflated and the ports are closed.
Second stage: the surgeon takes place in between the legs, the first assistant on the right side of the patient, the second assistant on the left side of the patient (Figure 16). Instead of a second assistant, a table-mounted laparoscopic liver retractor can be used for retraction. Typically five abdominal ports are placed in a similar fashion to the approach used for a laparoscopic Nissen fundoplication (Figure 3B). The liver is retracted medially and the dissection starts with transection of the gastro-hepatic ligament and transection of the right gastric artery using the harmonic scalpel. After opening the lesser sac the right pillar of the hiatus esophagei is identified and the gastro-esophageal junction is dissected out of the hiatus creating a window towards the greater sac behind the esophagus (Figure 17). From the lesser curvature side, the left gastric artery and vein are prepared and clipped at their base and transected taking all lymph nodes down to the offspring of the artery from the coeliac axis as to ensure complete lymph node clearance in along both vessels (Figure 18). The dissection continues along the greater curvature of the stomach taking great care not to damage the right gastro-epiploïc artery: the greater sac is incised and opened above the transverse colon and divided in the direction of the spleen. The short gastric vessels are divided and the dissection continues until the fundus is completely freed and the hiatus and left pillar are coming in to view. This is followed by the mobilization of the distal part of the greater curvature always away from the gastro-epiploic artery close to the transverse colon. In skinny patients care is taken not to open and erroneously divide the mesenterium of the transverse colon. All adhesions between the posterior gastric wall and pancreas are freed until the pylorus is reached. During the entire dissection process tissues are grasped only on the lesser curvature which later on will be resected so to avoid traumatization of the tissues of what will become the gastric tube (Figure 19). The gastric tubulation is performed using endostaplers introduced from the right upper quadrant, after having the liver retractor placed in the subxiphoidal position during tubulation. The first stapler is placed at the lesser curvature about 5 cm proximally from the pylorus at the level of the crow’s foot to achieve a gastric tube of 4 to 5 cm width. Of course the nasogastric tube has been pulled back beforehand. The gastric tube is most of the times at least 30 cm long. No pyloric drainage procedure is performed (Figure 20). Now there is ample space to perform the lymph node dissection along the common hepatic artery, the splenic artery and the base of the coeliac axis to achieve a DII lymphadenectomy (Figure 21).
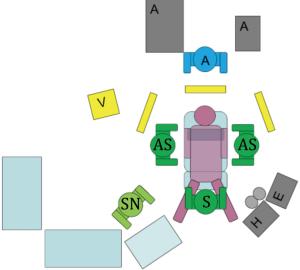





The esophagus is than dissected in the hiatus with en bloc resection of the peri-esophageal tissue till the chest cavity is entered, freeing completely the esophagus. At this time, it can be necessary to lower the intra-abdominal pressure or to put the chest drains on active suction in order to prevent intrathoracic overpressure.
To avoid loss of pressure due to the open communication between abdomen and chest through the hiatus, the proximal gastrectomy piece is used to plug the hiatus, allowing the intra-abdominal pressure to remain high enough as to ensure good visualization during the lymph node dissection. The gastric tube is than fixed to the proximal gastrectomy specimen using two temporary stitches.
Third stage: (Figure 22): an 8 cm long left cervical incision anterior to the sternocleidomastoid muscle and finishing in the suprasternal notch is performed. Omohyoid and strapped muscles are transected. Dissection is performed medially from the great vessels and laterally from the thyroid gland clipping and transecting eventually its feeding arteries. During this procedure, again great care is taken not to damage the left recurrent nerve. The cervical esophagus is then mobilized until the thoracic part of the dissection is reached. The esophagus with the proximal gastrectomy specimen can be exteriorized through the neck incision thus pulling up the gastric conduit during temporary ventilation stop and under direct laparoscopic vision in order to prevent torsion. At this point a classic end-to-side anastomosis is performed: we prefer a semi-mechanical anastomosis, using an endostapler for the posterior wall of the esophagogastric anastomosis and finishing the anterior part of the anastomosis using a double layer continuous stitch after transection and resection of the esophagus. The nasogastric tube is subsequently repositioned into the gastric conduit under direct visual control before finishing the anterior part of the anastomosis. The anastomosis is then reduced from the neck into the thoracic inlet behind the trachea. The neck incision is closed after placement of a closed suction drain system.

Finally the gastric tube is then retracted towards the abdomen and fixed to the hiatus using separate stay sutures as to avoid intrathoracic migration of abdominal organs.
If deemed necessary, a feeding jejunostomy can be placed.
Trocars are removed under visual control, pneumoperitoneum is deflated and incisions are closed. A pyloric drainage procedure is not routinely performed. No drain is left in the abdominal cavity.
Role of team members
Surgeon, first assistant, scrub nurse and anesthesiologist are all of pivotal importance during this complex and sometimes challenging procedure. The individual tasks of each team member are already described in the text. If a new team member is not familiar with the procedure, an experienced colleague will help and assist during the procedure (and during the learning curve) and this works for all team members.
Post-operative management
To optimize the results of surgery, this type of procedure should be performed in an appropriately equipped center that is familiar with the meticulous management of the postoperative course. If necessary, bronchoscopy or eventually a mini-tracheostomy placed either at the time of surgery or sometimes thereafter can be used to ensure adequate bronchial toilet. In the postoperative management, the role of the physiotherapist is crucial in order to prevent pulmonary complications.
Fluid balance and oxygen saturation should be closely monitored and oxygen supplementation is mandatory. Fluid restriction is essential to avoid cardiac and respiratory complications. It is also vital to maintain adequate and balanced nutrition during the whole postoperative period and therefore total parenteral nutrition or enteral feeding by jejunostomy, when placed at the time of surgery, is given.
Thrombosis prophylaxis is continued by sequential pneumatic compression devices for the first two postoperative days and subcutaneous injection of low molecular weight heparins. Prophylactic antibiotics are given for 24 hours.
Physiotherapy with gradual breathing exercises and general condition exercises is performed from the day of surgery to the day of discharge at least twice a day.
Since it is essential to avoid stasis in the gastric tube and subsequent respiratory complications secondary to aspiration, the nasogastric tube is kept in place and gastroprokinetic drugs (domperidon and erythromycin) are administered.
In case these preventive measures are of no avail and gastric stasis and subsequent gastric dilation occur, endoscopic balloon dilation of the pylorus will be performed.
A contrast study to check the integrity of the anastomosis in case of cervical location is not routinely performed. At day 5, if there is no clinical signs of anastomotic leak, oral fluids are started. In case of intrathoracic anastomosis a gastrografin swallow will be performed at day 5 and oral fluid and soft diet are started if no leak is visualized. On the same day the epidural catheter is removed and the patient is encouraged to mobilize fully. Oral analgetics are administered lege artis. The chest drain will be removed when the effluent amounts to less than 300 mL of fluids. Patients are discharged when they are able to tolerate the soft diet and the pain is sufficiently controlled to permit normal mobilization. The patient is than seen in the outpatient clinic one month after discharge.
Tips, tricks and pitfalls
- Patient selection is crucial;
- This procedure should be performed in high volume centers by an experienced team that can also offer alternative treatment possibilities;
- Correct patient installation together with the anesthesiological team is pivotal;
- Gentle tissue handling, especially using a no-touch technique for the gastric tube;
- If a bleeding occurs during the procedure, compression allows to evaluate the situation and can also be the sole treatment;
- In case of doubts, conversion is the safest option.
Where are we going from here?
Today esophagectomy whether open or with MIE can be performed with a minimum of blood loss and blood transfusion is only needed in a minority of patients. MIE is thought to have a positive impact on pain resulting in less need for analgesia, less pulmonary complications and shorter ICU stay.
All incremental steps of progress have resulted in reduced postoperative mortality and morbidity and thus better oncological outcome.
Despite all efforts, nowadays one in two patients will still die from recurrence and postoperative morbidity still occurs in one out of three patients.
New technologies, techniques and treatment modalities are emerging in an ongoing search to improve results. Experience with robotic esophagectomy and uniportal VATS is growing reportedly reducing even more postoperative complications and related morbidity (7).
Sentinel lymph node mapping and navigational techniques are tested hoping to avoid in the future the surgical trauma created by extensive lymphadenectomy in selected patients.
Treatment modalities aiming at preserving the esophagus such as EMR for early T1 cancers and definitive chemoradiation are already in place and in some centers definitive chemoradiation is the standard of care for advanced squamous cell carcinoma. These patients are closely monitored and in case of local recurrence salvage esophagectomy is offered as the last option in selected patients.
A wide spectrum of biologicals in the search for a more targeted therapy based on individual genetic profiles of the cancer is promising a tailored individualized treatment.
Interventional endoscopic techniques are booming stimulated by the ever increasing miniaturization of instrumentation. The recent introduction of POEM challenging the classic laparoscopic Heller myotomy for achalasia is one perfect example of what can be expected in the future. Here the sky seems to be the limit, not to speak about the perspectives offered by the nanotechnology both in the area of diagnostics as well as in the therapeutic arena.
From all this it must become clear that there is a definite need for superspecialisation in the field of esophageal pathology both benign and malignant.
The surgeon of tomorrow dealing with esophageal cancer will work in close collaboration with his/her peers in oncology, (interventional) radiology, interventional endoscopy….
This means that the different specialties as they are existing today will gradually grow towards each other eventually intertwining or merging. The result will be a complete re-engineering from surgery as a technical act into a unique form of therapy for each individual patient and for the better of the patient.
But this will require a strong engagement of from the surgeon to master the knowledge of esophageal cancer and to master the specifics of diagnosis and therapy (including complications and failure) of esophageal cancer.
Now is the time to act.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Supplementary
Card 1 (Figure S1)
- Equipment for first stage installation (Figure 2):
- Armed single lumen endotracheal tube with bronchus blocker;
- Nasogastric tube;
- Operating table with armrests;
- Bean bag;
- Pillows to support shoulders and pelvis;
- Foam protection for elbows and knees;
- Head support with integrated mirror;
- Two side supports fixed on the table;
- Warming blanket;
- Sequential pneumatic compression devices.
- Equipment for first stage surgical procedure:
- Endoscopic camera 10 mm, 30°;
- CO2 insufflation with Veress needle;
- Two 12 mm standard laparoscopy trocars and two 5.5 mm standard laparoscopy trocarts;
- Standard laparoscopy instruments: fenestrated clamps, electrocauter hook, scissors, dissection clamp;
- Additional laparoscopy instruments: 5 mm endopeanut, 5 and 10 mm vascular clips, 5 mm suction device and endoscopic vascular clamp;
- 45 mm vascular endostapler device;
- Backup standard thoracotomy set in case of conversion.
Card 2 (Figure S2)
- Equipment for installation in supine position (Figure 3):
- Operating table with leg supports;
- Bean bag;
- Pillow to put under the shoulders for neck hyperextension;
- Arm support along the body;
- Sequential pneumatic compression devices.
- Equipment for second stage surgical procedure:
- Endoscopic camera 10 mm, 30°;
- CO2 insufflation with Veress needle;
- Three 12 mm standard laparoscopy trocars and two 5.5 mm standard laparoscopy trocarts;
- Standard laparoscopy instruments: fenestrated clamps, electrocauter hook, scissors, dissection clamp;
- Additional laparoscopy instruments: 5 mm liver retractor, 5mm and 10mm vascular clips, ultrasonic dissection device, 5 mm suction device and laparoscopic needle holder;
- 45 mm endostapler device;
- Backup standard laparotomy set in case of conversion.
Card 3 (Figure S3)
- Equipment for third stage surgical procedure:
- Standard open instruments;
- Self-retaining Weitlaner retractor;
- Yankauer suction device;
- Polydioxanone 4/0 absorbable monofilament sutures;
- Polyglyconate 3/0 absorbable monofilament sutures;
- Polyethylene terephthalate 3/0 nonabsorbable polyfilament sutures;
- 45 mm endostapler device.
References
- Torek F. The first successful case of resection of the thoracic portion of the esophagus for carcinoma. Surg Gynecol Obstet 1913;16:614.
- De Bakey M, Ochsner A. Carcinoma of the esophagus. Postgrad Med 1948;3:192-8. [Crossref] [PubMed]
- Raymond DP, Seder CW, Wright CD, et al. Predictors of Major Morbidity or Mortality After Resection for Esophageal Cancer: A Society of Thoracic Surgeons General Thoracic Surgery Database Risk Adjustment Model. Ann Thorac Surg 2016;102:207-14. [Crossref] [PubMed]
- Surveillance, Epidemiology, and End Results Program. SEER Stat Fact Sheets: esophageal cancer. Available online: http://seer.cancer.gov/statfacts/html/esoph.html, accessed September 17, 2014.
- Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb 1992;37:7-11. [PubMed]
- Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg 2003;238:486-94; discussion 494-5. [PubMed]
- Qureshi YA, Dawas KI, Mughal M, et al. Minimally invasive and robotic esophagectomy: Evolution and evidence. J Surg Oncol 2016;114:731-735. [Crossref] [PubMed]
- Jang R, Darling G, Wong RK. Multimodality approaches for the curative treatment of esophageal cancer. J Natl Compr Canc Netw 2015;13:229-38. [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Nafteux P, Moons J, Coosemans W, et al. Minimally invasive oesophagectomy: a valuable alternative to open oesophagectomy for the treatment of early oesophageal and gastro-oesophageal junction carcinoma. Eur J Cardiothorac Surg 2011;40:1455-63; discussion 1463-4. [PubMed]
- Chapter 1: changing trends and lessons learned from 2500 esophagectomies. In: Nafteux P. The continuous quest for quality improvement after esophagectomy for cancer. Leuven: Thesis Academic press, 2016:16-42.
- Markar SR, Wiggins T, Antonowicz S, et al. Minimally invasive esophagectomy: Lateral decubitus vs. prone positioning; systematic review and pooled analysis. Surg Oncol 2015;24:212-9. [Crossref] [PubMed]
- Tanaka E, Okabe H, Kinjo Y, et al. Advantages of the prone position for minimally invasive esophagectomy in comparison to the left decubitus position: better oxygenation after minimally invasive esophagectomy. Surg Today 2015;45:819-25. [Crossref] [PubMed]
- Shen Y, Feng M, Tan L, et al. Thoracoscopic esophagectomy in prone versus decubitus position: ergonomic evaluation from a randomized and controlled study. Ann Thorac Surg 2014;98:1072-8. [Crossref] [PubMed]
- Logan A. The surgical treatment of carcinoma of the esophagus and cardia. J Thorac Cardiovasc Surg 1963;46:150-61. [PubMed]
- Skinner DB. En bloc resection for neoplasms of the esophagus and cardia. J Thorac Cardiovasc Surg 1983;85:59-71. [PubMed]
- Akiyama H, Tsurumaru M, Kawamura T, et al. Principles of surgical treatment for carcinoma of the esophagus: analysis of lymph node involvement. Ann Surg 1981;194:438-46. [Crossref] [PubMed]
- Orringer MB, Sloan H. Esophagectomy without thoracotomy. J Thorac Cardiovasc Surg 1978;76:643-54. [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 2008;248:549-56. [PubMed]
- Lerut T, Nafteux P, Moons J, et al. Three-field lymphadenectomy for carcinoma of the esophagus and gastroesophageal junction in 174 R0 resections: impact on staging, disease-free survival, and outcome: a plea for adaptation of TNM classification in upper-half esophageal carcinoma. Ann Surg 2004;240:962-72; discussion 972-4. [Crossref] [PubMed]
- Depypere L, Coosemans W, Lerut T, et al. Start dissection. Asvide 2017;4:081. Available online: http://www.asvide.com/articles/1389
- Depypere L, Coosemans W, Lerut T, et al. Azygos. Asvide 2017;4:082. Available online: http://www.asvide.com/articles/1390
- Depypere L, Coosemans W, Lerut T, et al. Pars membranacea. Asvide 2017;4:083. Available online: http://www.asvide.com/articles/1391
- Depypere L, Coosemans W, Lerut T, et al. Identification L recurrent nerve. Asvide 2017;4:084. Available online: http://www.asvide.com/articles/1392
- Depypere L, Coosemans W, Lerut T, et al. Aorta + subcarinal. Asvide 2017;4:085. Available online: http://www.asvide.com/articles/1393
- Depypere L, Coosemans W, Lerut T, et al. Thoracic duct supradiaphragmatic. Asvide 2017;4:086. Available online: http://www.asvide.com/articles/1394
- Depypere L, Coosemans W, Lerut T, et al. Paratracheal. Asvide 2017;4:087. Available online: http://www.asvide.com/articles/1395
- Depypere L, Coosemans W, Lerut T, et al. Right recurrent. Asvide 2017;4:088. Available online: http://www.asvide.com/articles/1396
- Depypere L, Coosemans W, Lerut T, et al. Left recurrent. Asvide 2017;4:089. Available online: http://www.asvide.com/articles/1397
- Depypere L, Coosemans W, Lerut T, et al. Overview final. Asvide 2017;4:090. Available online: http://www.asvide.com/articles/1398
- Depypere L, Coosemans W, Lerut T, et al. Lesser curvature. Asvide 2017;4:091. Available online: http://www.asvide.com/articles/1399
- Depypere L, Coosemans W, Lerut T, et al. Left gastric artery. Asvide 2017;4:092. Available online: http://www.asvide.com/articles/1400
- Depypere L, Coosemans W, Lerut T, et al. Greater curvature. Asvide 2017;4:093. Available online: http://www.asvide.com/articles/1401
- Depypere L, Coosemans W, Lerut T, et al. Gastric tube. Asvide 2017;4:094. Available online: http://www.asvide.com/articles/1402
- Depypere L, Coosemans W, Lerut T, et al. Lymph node dissection. Asvide 2017;4:095. Available online: http://www.asvide.com/articles/1403
- Depypere L, Coosemans W, Lerut T, et al. Anastomosis. Asvide 2017;4:096. Available online: http://www.asvide.com/articles/1404
Cite this article as: Depypere L, Coosemans W, Nafteux P, Van Veer H, Neyrinck A, Coppens S, Boelens C, Laes K, Lerut T. Video-assisted thoracoscopic surgery and open chest surgery in esophageal cancer treatment: present and future. J Vis Surg 2017;3:30.