3D CT simulation in minimally invasive thoracic surgery
Introduction
In 1970’s, the technology of computed tomography was developed by Sir Godfrey N. Hounsfield based of the theory of Alain Cormack who worked for EMI and they were awarded Novel Prize in 1979. The influence of CT was huge and comprehensive that would change the treatment method in all medical fields.
Notable progress was accomplished in the history of CT in 1990’s. “The helical CT” technology in which detector and the X-ray tubes could turn and correct the patient data continuously was introduced 20 years after the invention of the CT. Then 10 years later, multi-detector scanning CT was developed. Both of these technologies could provide volume data of the objects, especially the technology based on Multi detector CT which appeared around 2000 further emphasize the aspect of three-dimensional image technology.
In addition to the evolution of hardware, new methods were brought about by advancement of the image processing technology; “surface rendering” and “reconstruction of the CT” as methods for stereoscopically reconstruct the CT volume data. In both technology, three-dimensional (modeling) image processed by the raw volume CT data, subsequently process these data stereoscopically with a two-dimensional image (rendering). There is a difference in expression method between aforementioned two, and a method of seeing the surface of the mass with a certain threshold is called a “surface rendering method”. “Volume rendering method” enables observation of the inside of the objects by changing opacity of the different layers. Surface rendering modeling realized visualization of vessels called CT angiography (CTA). Real-time rendering processing had been difficult but with the progression of the hardware, both real-time rendering image processing has been realized. Starting from simple processing software such as NIH image run on the Macintosh, developed by Wany Rasband, various high-performance software has been available with high level function which enables calculation of volume, automatic measurement of blood vessel diameter, recognition and separation of internal organs. Furthermore, high-performance software such as AZE Virtual PlaceTM or Synapse Vincent [2008] have brought about automatic separation and visualization of lung lobes, segmentation (1,2). Here we introduce some images and movies which describe the features of surgery using this relatively new technology in the field of minimally invasive thoracic surgery.
Lobectomies and vessel identification by CTA
Lung surgery is very unique in that the targeted organ collapse and deform in great extent during the procedures. And in the minimally invasive thoraco-scopic settings, the approach is limited from the ports, which results in the limitation of observation. For this reason, it is very important to understand the branching form of blood vessels before surgery for safety and efficiency. In this way, CTA technology through which one can observe blood vessel branching noninvasively had attracted great attention since its beginning of the multi-detector CT and applied in various ways in practice.
Recent advancement of the technology enabled rapid identification of the pulmonary artery and vein by simple clicking the main trunks of pulmonary artery and left atrium, thus surgeon can easily observe the branching of the blood vessels and grasp the anatomical features of the case before the procedures (3,4). Some program can process the vessel images, separate pulmonary artery and vein automatically even from non-contrast enhanced study although the quality and accuracy is compromised comparing to the CE study (2,5).
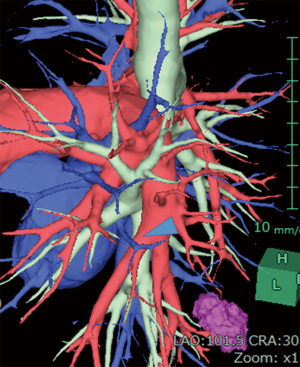
In the upper lobe lobectomy of the both side, special care is required in identification of the arteries because the variation of the arteries is frequent. In the left lung PA branching, two to seven branches may originate from the proximal PA to supply the left upper lobe and lingual segment. The most common variation is lingular branch of the PA; 70% to 90% of the arteries for lingual segments arise from the left interlobar artery and the rest arise from the common branch for the anterior and apico-posterior segment of the upper lobe (6). Surface rendering images are quite useful to grasp the anatomy of the pulmonary artery (1,7). In the Figure 1, a surface rendering vessel image clearly shows that the lingual branch shares common root with the artery to the anterior and the apical segment. Preoperative understanding of the branching pattern may avoid an injury of the artery and furthermore, may reduce the operation time. In this case, the interlobar fissure was incomplete. Since the lingular branch had been confirmed rising from the common root of the branch to the anterior segment, the pulmonary artery was dissected before completing the fissure. The interlobar fissure was made after all the arteries and the bronchus had dissected (Figure 2).

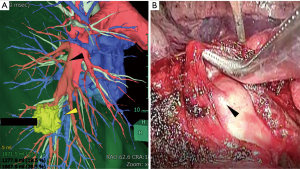
An aberrant branch to the apical segment of the right lower lobe is shown in Figure 3A; the artery was in the middle of the incomplete major fissure was clearly identified by the surface rendering model. In this case, preoperative image analysis clarified this rare variation and could avoid possible arterial injury by careless or indifferent procedure to complete fissure (Figure 3B).
Limitation of surface rendering images of vessels
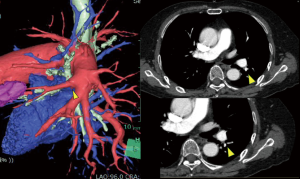
CTA of a patient with lung cancer in the left lower lobe was shown in Figure 4. In this case, a small branch of pulmonary artery around the bronchus of the lingual segment left unidentified at the time of operation resulted in the injury of PA and conversion to the thoracotomy (Figure 5). The identification rate of the branch of the pulmonary artery reportedly ranged from 95.1% to 98.7% (4,7,10). Artery with small caliber (smaller than 2 mm) tended to be unidentified, and identification rate was likely low in the branches of the right upper pulmonary artery (7). Some of the unidentified branches by the volume rendering could be detected by careful reading of 0.625 mm contrast enhanced study (7) and to get optimal result in processing the vascular images, careful timing and dose of the injection of the contrast medium is important (1,11).

Simulation and navigation for sublober resection of small pulmonary nodule
Recent advancement and prevalence in CT resulted in increasing number of patients with small or GGN lesion, and often they tend to have multiple or metachronous lesions (12). For such cases, sub-lobar resections are favorable both for oncological reasons and for the pulmonary functions preservation. For these patients, there developed several simulation software which process three dimensional images to find location of the target and indicate the precise safety margin from the cancer (13). Some software provided with the function which enables visualization of the intersegmental septa and, there are several reports of sublobar resections with the best use of this function (2,14). To address the difficulty to locate the target of the lung which deforms during the surgery, we have been proposing a technique called ‘Virtual Assisted Lung Mapping (VAL-MAP)’. We have reported the advantages of the combination of three-dimensional visualization and the dye marking to locate the target lesion and to identify the optimal resection line to secure the margin (15) .
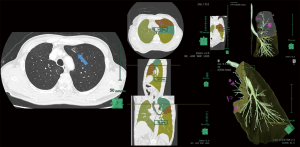
In the Figure 6 (Figure 7), the positions of the dye which was marked for the target (GGN lesion with 12 mm in its diameter) and the imaginary boundary for the resection line were shown. This patient had had the right upper lobe lobectomy few years ago, deemed to have metachronous lung cancer in his anterior segment of the left upper lobe. Because of the limited pulmonary reserve after contralateral lobectomy, sub-lobar resection was selected. Prior to the operation, 1 mL of indigo carmine was injected adjacent to the target lesion and to the additional three points which planned to show the boundary of the adequate surgical margin. An additional scan of CT to confirm the multiple markings on 3-dimensional virtual images was followed by the anterior segmentectomy of the left upper lobe. The great advantage of this method is that by referring the dye marking it is possible to secure the margin from the tumor pre- and intra-operatively.

Conclusions
Since the helical scan technology was introduced 25 years ago which enabled three-dimensional observation of the organ, evolution of the technology surrounding CT has enlarged its role from diagnostic modality to a tool which supports treatment more directly and has increased its importance. As a result of great advancement of hardware and image processing technology the simulation and even navigational surgery has started to be introduced in minimally invasive thoracic surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ikeda N, Yoshimura A, Hagiwara M, et al. Three dimensional computed tomography lung modeling is useful in simulation and navigation of lung cancer surgery. Ann Thorac Cardiovasc Surg 2013;19:1-5. [Crossref] [PubMed]
- Chen-Yoshikawa TF, Date H. Update on three-dimensional image reconstruction for preoperative simulation in thoracic surgery. J Thorac Dis 2016;8:S295-301. [PubMed]
- Akiba T, Marushima H, Harada J, et al. Importance of preoperative imaging with 64-row three-dimensional multidetector computed tomography for safer video-assisted thoracic surgery in lung cancer. Surg Today 2009;39:844-7. [Crossref] [PubMed]
- Fukuhara K, Akashi A, Nakane S, et al. Preoperative assessment of the pulmonary artery by three-dimensional computed tomography before video-assisted thoracic surgery lobectomy. Eur J Cardiothorac Surg 2008;34:875-7. [Crossref] [PubMed]
- Shimizu K, Nakano T, Kamiyoshihara M, et al. Segmentectomy guided by three-dimensional computed tomography angiography and bronchography. Interact Cardiovasc Thorac Surg 2012;15:194-6. [Crossref] [PubMed]
- Yamashita H. Roentgenologic anatomy of hilar markings. Rinsho Hoshasen 1976;21:929-31. [PubMed]
- Hagiwara M, Shimada Y, Kato Y, et al. High-quality 3-dimensional image simulation for pulmonary lobectomy and segmentectomy: results of preoperative assessment of pulmonary vessels and short-term surgical outcomes in consecutive patients undergoing video-assisted thoracic surgery†. Eur J Cardiothorac Surg 2014;46:e120-6. [Crossref] [PubMed]
- Sato T, Date H. Robot assisted left lower lobectomy, the case presented in . Incomplete fissure between left upper and lower lobe was made after pulmonary artery and bronchus for left lower lobe had been divided. Asvide 2017;4:078. Available online: http://www.asvide.com/articles/1385
- Sato T, Date H. Movie of VATS left upper lobectomy, the case presented in which was converted to open thoracotomy to fix the injury of the small branch of the lingular artery. Asvide 2017;4:079. Available online: http://www.asvide.com/articles/1386
- Nagashima T, Shimizu K, Ohtaki Y, et al. An analysis of variations in the bronchovascular pattern of the right upper lobe using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg 2015;63:354-60. [Crossref] [PubMed]
- Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 2010;256:32-61. [Crossref] [PubMed]
- Committee for Scientific Affairs, Sakata R, Fujii Y, et al. Thoracic and cardiovascular surgery in Japan during 2009: annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2011;59:636-67. [Crossref] [PubMed]
- Yamanaka J, Saito S, Fujimoto J. Impact of preoperative planning using virtual segmental volumetry on liver resection for hepatocellular carcinoma. World J Surg 2007;31:1249-55. [Crossref] [PubMed]
- Saji H, Inoue T, Kato Y, et al. Virtual segmentectomy based on high-quality three-dimensional lung modelling from computed tomography images. Interact Cardiovasc Thorac Surg 2013;17:227-32. [Crossref] [PubMed]
- Sato M, Omasa M, Chen F, et al. Use of virtual assisted lung mapping (VAL-MAP), a bronchoscopic multispot dye-marking technique using virtual images, for precise navigation of thoracoscopic sublobar lung resection. J Thorac Cardiovasc Surg 2014;147:1813-9. [Crossref] [PubMed]
- Sato T, Date H. Pre-operative assessment of the case shown in the . Asvide 2017;4:080. Available online: http://www.asvide.com/articles/1387
Cite this article as: Sato T, Date H. 3D CT simulation in minimally invasive thoracic surgery. J Vis Surg 2017;3:26.


