Video-assisted and minimally-invasive open chest surgery for the treatment of mediastinal tumors and masses
Introduction
Because of significant technological developments, widespread availability of video imaging, and better instrumentation, the use of minimally invasive approaches for the surgical treatment of mediastinal tumors and masses became popular in the early 1990s. This popularity changed the pattern of practice of most thoracic surgeons, and over the subsequent years, video-assisted thoracic surgery (VATS) found more and more applications and indications.
It is well recognized that VATS plays an important role in the diagnostic evaluation of some patients with mediastinal tumors and masses because it provides easy access to all mediastinal compartments. It is also well accepted that VATS resection of benign lesions such as mediastinal cysts and most neurogenic tumors is both feasible and safe because these lesions are nearly always benign and well delineated in addition to having relatively sparse vasculature.
The role of VATS in the surgical management of malignant neoplasms of the mediastinum such as thymic epithelial tumors (TETs) is, however, more controversial because several surgeons have expressed concerns about the ability of thoracoscopic procedures to maintain adherence to sound surgical oncologic principles.
In this article, we first describe the anatomy of the mediastinum and then review indications, limitations, techniques, and results of VATS as well as of other minimally-invasive open chest surgery approaches for the surgical management of mediastinal tumors and masses. To do so, the article was purposely written by two surgeons with vastly different backgrounds and thoracic surgical experience. One of them (GR), a younger thoracic surgeon, is very familiar with VATS approaches and technologies while the other (JD), a now retired thoracic surgeon, is relatively unfamiliar with these techniques. This combination of authorship appears ideal to analyze the pros and cons of the use of minimally-invasive approaches in the surgical management of mediastinal tumors and masses.
Anatomic divisions of the mediastinum
Because specific lesions have a predilection for certain sites, dividing the mediastinum into different compartments is helpful. It is also of practical value when one is selecting the best surgical approach for a given mediastinal mass or tumor. Classically, the mediastinum is divided into four compartments (four compartment scheme): superior, anterior, middle, and posterior (1,2) (Figure 1) .
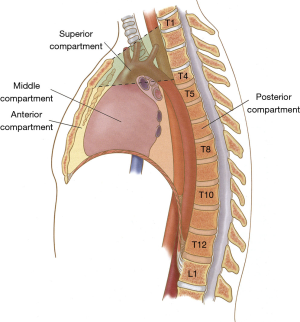
In that scheme, the superior mediastinum originates at the thoracic inlet and extends inferiorly to a horizontal plane established by a virtual line drawn between the sterno-manubrial junction (angle de Louis) in the front and the lower part of the fourth thoracic vertebra (T4) in the back. This compartment contains all of the structures traversing the thoracic inlet, including the aorta and its major branches, the intrathoracic trachea, the upper third of the thoracic esophagus, the upper half of the superior vena cava, and the upper poles of the thymus.
The anterior mediastinum (antero-inferior), which is the smallest of this 4-compartment scheme, is located between the body of the sternum in the front and the anterior pericardium posteriorly. It contains loose connective tissues, mediastinal fat, and the body of the thymus.
The middle mediastinum is bounded anteriorly and posteriorly by the pericardium (Figure 1) and it contains the pericardium itself, the intrapericardial cardiovascular structures, the carina and both main bronchi, and important lymph nodes located at and around the carina.
The posterior mediastinum extends from the dorsal surfaces of the pericardium, tracheal bifurcation, and main pulmonary blood vessels to the ventral surface of the lower eight thoracic vertebrae. It contains the esophagus, descending thoracic aorta, azygos vein, thoracic duct, and sympathetic chains.
VATS and minimally-invasive open chest surgery for tumors and masses of the antero-superior mediastinum
Common causes of tumors and masses located in the antero-superior mediastinum include TETs, germ-cell tumors, and rare mesenchymal neoplasms. On occasion, ectopic parathyroid tissue can also be found in this location. The surgical management of these lesions has always presented a challenge to thoracic surgeons (3), a challenge which has become more significant since the advent of minimally-invasive approaches.
TETs
General considerations
TETs include thymomas and thymic carcinomas (4-6). The majority of thymomas tend to be slow-growing tumors which seldom metastasize but local invasion into surrounding structures such as the pericardium, heart, and veins of the anterior mediastinum is not uncommon. In contrast, thymic carcinomas are more aggressive lesions associated with much poorer prognosis.
TETs are usually staged according to the Masaoka Staging System (7). Stage 1 tumors are completely encapsulated lesions often referred to as “benign tumors”. Tumors are classified as stage II if they have microscopically penetrated through the capsule or invaded the surrounding mediastinal fat. Stage III tumors are those which have invaded neighboring structures (gross or microscopic invasion) while stage IVa tumors represent lesions with intrathoracic spread (usually pleural) and stage IVb tumors with distant metastases.
The mainstay of treatment of most TETs is total surgical removal of the tumor and complete excision of the thymus gland. Well accepted prognostic factors and predictors of survival after resection include completeness of resection, stage, histological subtype, and size (8,9).
Controversies in surgical management
The traditional thinking with regards to thymectomy is that the operation should be carried out through a median sternotomy. It is argued that this approach not only allows for more complete resection of the thymus gland including its upper poles but that it also lowers the risks of positive resection margins or capsular rupture. Direct tumor invasion into pericardium, phrenic nerves, pleura, lungs, superior vena cava, or innominate vein, which may have been overlooked pre-operatively, can also be dealt with understanding that doing a complete resection is the ultimate goal of surgery (10-12). Many experienced thoracic surgeons, including the senior author of this review, also argue that the operation done through a median sternotomy is well tolerated and does not cause much more post-operative pain and is not associated with longer lengths of stay than the same operation done through minimally invasive approaches.
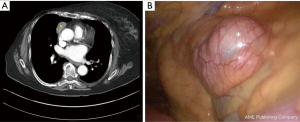
Thymectomy done for early-stage (stages I and II) TETs (Figure 2) through minimally invasive approaches also appears to be both feasible and safe (13-16). Proponents of this strategy argue that such approaches are associated with less operative trauma and chest wall disruption, reduction in pulmonary or other morbidities, reduction in post-operative pain, and shorter length of stay. Pain reduction is particularly important in patients with associated myasthenia gravis (MG) because these individuals are prone to post-operative respiratory complications secondary to muscle weakness and immunosuppression. In cohort analysis, all retrospective, it also appears that oncologic outcomes are comparable between sternotomy and VATS groups during short or intermediate-term follow-up. It is, however, important to remember that the generally indolent nature of stages 1 and II TETs requires long-term follow-up before significant differences between treatment groups can be observed.
A second controversy concerns the extent of thymic resection required to achieve optimal oncological outcomes. This controversy is, in part, due to the lack of proper definitions as to the meaning of “complete, extended, or maximal thymectomy (17)” and to the fact that some authors have suggested that sub-total thymectomy may be adequate surgery for the treatment of early stage thymomas (18). Not surprisingly, this controversy has mostly come up since the advent of minimally invasive approaches. Despite these reports, the great majority of surgeons still recommend that thymic tumors be treated by complete thymectomy with removal of the upper poles and surrounding mediastinal fat as described by Port and Ginsberg in 2001 (19). Most surgeons believe that complete thymectomy is associated with better overall survival, significant reduction in the incidence of local recurrences, and, likely, lower incidence of post-operative MG (14).
The extent of lymph node sampling and need for lymph node dissection is also a matter of some controversy. Traditionally, it was believed that the incidence of lymph node metastases was very low in TETs and that these patients did not require lymph node dissection, especially if they had small size (less than 6 cm) and early stage tumors . This has certainly been the experience of the senior author of this review. More recent data from the SEER database (20) have shown, however, that as much as 15% of patients may have lymph node involvement and that all patients undergoing thymectomy for TETs should have routine sampling or even dissection of lymph nodes, especially of the nodes located in the anterior mediastinum (17,20).
Thoracoscopic techniques
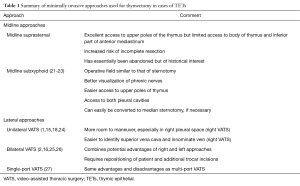
There is a wide variety of minimally invasive technical approaches that have been described for thymectomy and these are summarized in Table 1. Depending on the experience of individual surgeons as well as of tumor location, a combination of these minimally invasive approaches can be used. Thoracoscopic approaches, for instance, can be combined with cervical or subxiphoid incisions.
Full table
Robotic thymectomy has also been reported (28-31), mostly for early stage thymomas. The technique allows for the use of articulated instruments which can be useful when dissecting the upper poles of the thymus or in areas where the large veins of the anterior mediastinum may be susceptible to injury. The theoretical advantages of robotics have not yet, however, translated into clear-cut clinical benefits over other minimally invasive approaches. In addition, they come at the expense of increased operative times and operative costs.
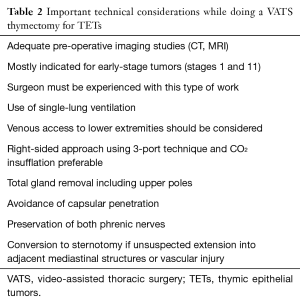
The most important technical considerations for those doing this type of surgery are summarized in Table 2. The first such consideration is that adequate preoperative imaging studies (CT, MRI) are imperative to determine the accessibility of the lesion and to determine possible tumor invasion of neighboring mediastinal structures that would contraindicate the use of minimally-invasive approaches (Figure 3). This is especially true if patients with locally advanced tumors have had induction therapies in order to improve resectability (32).
Full table
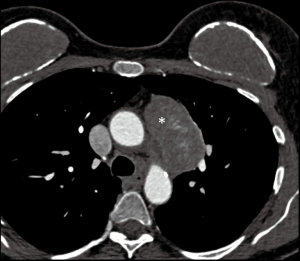
In order to have a clear operative field, single-lung ventilation is relatively standard. We also believe that it is wise to secure venous access to lower extremities which could be useful in the eventuality of intraoperative vascular injury with significant bleeding and requiring urgent conversion to sternotomy.
Although the choice of using a left-or right-sided VATS approach depends on tumor location, most surgeons prefer a right-sided approach not only because the right pleural cavity is larger than the left but also because there is less interference from the heart, thus optimizing operative exposure. In addition, the superior vena cava and origin of the innominate vein are better visualized from the right which minimizes the risks of injury. Most surgeons use a 3-port technique with CO2 insufflation as an adjunct to single-lung ventilation.
As previously discussed, complete thymectomy should be carried out with an en-bloc dissection technique and the resection must include that of the upper thymic poles and adjacent mediastinal fat. It is imperative that one avoids capsular rupture with potential seeding of tumor cells in surrounding tissues because such rupture is associated with a significant incidence of local recurrences sometimes occurring after prolonged disease-free intervals. The phrenic nerves must absolutely be identified and preserved from injury, understanding that the contralateral nerve may be difficult to visualize if one is using a unilateral VATS approach. Although sacrifice of one phrenic nerve is generally well tolerated, bilateral phrenic nerve sacrifice is almost incompatible with normal life.
Particular care should be applied to minimize the risk of injury to the innominate vein but if this should occur, conversion to sternotomy must urgently be carried out, remembering that conversion to an open approach is not a sign of weakness but rather indicative of good judgment. Conversion to sternotomy should also be contemplated in cases where the surgeon documents previously unsuspected venous involvement requiring venous resection and reconstruction.
Ectopic parathyroid tissue
General considerations
Mediastinal surgery is sometimes indicated for the excision of intrathoracic ectopic parathyroid tissue associated with hyperparathyroidism or of ectopic mediastinal parathyroid adenomas (approximately 15–20% of all parathyroid adenomas). Ectopic parathyroid tissue is usually found in the anterior mediastinum near or within the thymus because both thymus and parathyroids share a common embryologic origin from the third and fourth bronchial pouches. Surgically, they are readily accessible through the neck or through VATS approaches (33-38).
In all cases, preoperative localization is mandatory because these tissues can be difficult to find at operation. In most cases, a combination of CT, MRI, and nuclear scanning with 99mTc-sestamibi offers a localization rate of 90–100%. Intraoperative navigation techniques including the use of a Gamma probe (33,37) or methylene blue (39) have also been advocated for successful excision of parathyroid adenomas.
Surgical approach and thoracoscopic techniques
Exploration of the anterior mediastinum for ectopic parathyroid tissue with or without adenoma has traditionally been carried out through a cervical incision or a manubrio-sternotomy (36-38). Depending on location, these can also be accessed through mediastinoscopy or parasternal anterior mediastinotomy.
More recently, the use of minimally-invasive approaches has gained in popularity because they are associated with decreased morbidity, faster recovery (35), and shorter postoperative length of stay (34). The side of the approach is determined by ectopic tissue or adenoma location and three ports are generally used. Low pressure (8–10 mmHg) CO2 insufflation may be helpful to improve surgical exposure.
Successful parathyroidectomy should always be confirmed intraoperatively by frozen sections and decline in parathormone levels by at least 50%. Normalization of blood calcium levels occurs at around 48–72 hours postoperatively. Overall cure rates range from 75% to more than 95%.
Germ cell tumors and other rare mesenchymal neoplasms
Approximately 5–10% of germ cell tumors arise within the antero-superior mediastinum (40) and this extra-gonadal location relates to migration failure of pluripotent cells along the urogenital ridge during embryogenesis. Germ cell tumors can be seen in all age groups although they are most often diagnosed in young adults with approximately half the patients being asymptomatic at diagnosis. On occasion, benign mature teratomas will rupture into the lung, pleural space, or even in the superior vena cava (41).
Surgical resection is the appropriate strategy for all cases of benign mediastinal teratomas. Because surrounding tissues are not infrequently adherent to the lesion, most teratomas should be resected through an open approach, either a sternotomy or lateral thoracotomy rather than through minimally-invasive approaches. Thoracoscopic resection should only be done for smaller lesions measuring less than 4 cm in diameter.
There is no role for surgical resection in patients with primary mediastinal seminomatous germ cell tumors because complete response to cisplatin-based chemotherapy is predictable. Residual mass after chemotherapy needs not to be resected because this tissue is nearly always benign. For patients with primary mediastinal non-seminomatous germ cell tumors, residual masses after chemotherapy may need to be resected (42), but because of the complexity of those cases as well as tissue alterations secondary to chemotherapy, these operations should always be done through an open approach, generally a sternotomy.
Primary mediastinal mesenchymal tumors are exceedingly rare and they can develop from adipose tissue, connective tissue, blood and lymph vessels, striated and smooth muscle, or any combination of these tissue. Because most such tumors are highly malignant with extensive local invasion, surgical resection is seldom indicated for their management.
VATS and minimally invasive open surgery for tumors and masses of the middle mediastinum
Lymph node masses and neoplasms
Although most types of lymphomas can potentially involve the mediastinum, only a few present as isolated mediastinal masses. If this diagnosis is suspected, generous tissue biopsy is essential not only to establish a diagnosis but also to properly sub-type the lymphoma according to the World Health Organization classification system. Adequate tissue can sometimes be obtained by percutaneous biopsy although this method is generally insufficient because determination of architectural pattern is necessary for lymphoma sub classification. If an open biopsy is necessary, it can be done through mediastinoscopy, mediastinotomy, and, on rare occasions, through a VATS approach. Surgery has no role in the management of patients with mediastinal lymphomas.
Bronchogenic cysts
General considerations
Bronchogenic cysts are closed sacs believed to be the result of an abnormal budding process that occurs during the early development of the foregut (43,44). Most of them are located in the middle mediastinum where they are closely related to the tracheobronchial tree. The majority of bronchogenic cysts seen in the adult population are asymptomatic but they often enlarge over time and can cause pressure on contiguous structures. Bronchogenic cysts can also be the site of complications such as infection and airway fistulisation. Although there is no consensus on the management of bronchogenic cysts, most surgeons advocate early removal because the majority of such cysts will ultimately become symptomatic or complicated, at which time operation is technically more difficult and hazardous (43,45).
Surgical approach and thoracoscopic techniques
In the past, bronchogenic cysts were resected through an open right-sided thoracotomy but VATS has now become the standard approach (44-49) unless the cyst is complicated. Interestingly, bronchogenic cysts have also been successfully resected through mediastinoscopy (50).
Before doing a VATS excision of mediastinal bronchogenic cysts, MRI is the most accurate imaging technique to delineate cyst anatomy and plan the surgery. Endoscopic ultrasonography may also provide useful information on the relationship between the cyst and surrounding structures.
The procedure is done under general anesthesia and selective one-lung ventilation and we recommend using a 3-port technique where port position varies according to cyst location. One key consideration is that the cyst must be completely resected because of the possibility of recurrence if it is not. If the cyst is found to be bulky and difficult to handle, intraoperative cyst aspiration can be done in order to facilitate its removal (51). If the cyst is found to be inflamed or densely adherent to the airway or surrounding vascular structures making complete resection unsafe, its epithelial lining can be destroyed by cautery or argon beam coagulation. One should not hesitate, however, to convert to open thoracotomy if there are dense adhesions between the cyst and surrounding structures or in cases where the airway is injured during operation.
Pleuro-pericardial cysts
Pleuro-pericardial cysts are thought to result from failure of fusion of one of the mesenchymal lacunae that normally fuse to form the pericardial sac. Most are located along the right cardiac border and are asymptomatic. If surgical resection becomes indicated, either to confirm diagnosis or for treatment of rarely occurring complications such as hemorrhage into the cyst or compression of the adjacent heart, lung or airway, it is most often done through a thoracoscopic approach (52,53).
VATS and minimally-invasive open chest surgery for tumors and masses of the posterior mediastinum
Neurogenic tumors
General considerations
Most intra-thoracic neurogenic tumors are located in the paravertebral sulci, both right and left. On occasion, they may originate from the phrenic or vagus nerves and will then be located in the middle mediastinum. Less than 10% of neurogenic tumors seen in the adult are malignant, but nearly half are in children (54-56). In approximately 10% of cases, neurogenic tumors will extend into the spinal canal and such lesions are called dumbbell or hourglass tumors.
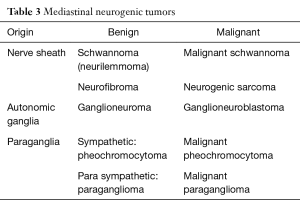
Neurogenic tumors may have one of three origins, the nerve sheath, the autonomic ganglia, and the paraganglia (Table 3). Although observation alone is a reasonable course of action for tumors that have been stable for years, the majority of neurogenic tumors should be resected because of uncertain diagnosis, location near or into the spinal canal or possible malignancy of the lesion.
Full table
When surgery is contemplated, preoperative imaging with MRI is mandatory because this technique best defines possible tumor extension into the spinal canal (57,58).The role of thoracic aortography to document the site of origin of the artery of Adamkiewicz, which supplies the anterior spinal artery, is controversial. Some surgeons, particularly those in Europe, always perform thoracic aortography before surgery, and if the artery of Adamkiewicz is found to arise in the area of the tumor, excision is not recommended unless absolutely necessary. If the artery is found to arise at a distance from the tumor, surgery can proceed without concern for possible damage to the blood supply of the spinal cord (54).
Surgical approach and thoracoscopic techniques
In the past, surgical resection of neurogenic tumors of the posterior mediastinum was carried out through a standard postero-lateral thoracotomy (56,59,60). The use of a VATS approach was first reported in 1992 by Landreneau et al. (61) and since then, it has become the preferred technique (62-68) because it greatly improves operative visualization making surgery easier and safer. In addition, VATS reduces the amount of postoperative pain and shoulder girdle dysfunction resulting in better functional outcomes (57,69). Neurogenic tumors are indeed ideally suited for VATS resection because the majority are small and well encapsulated (Figure 4).
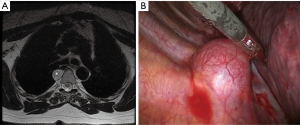
The operation is usually done under one-lung ventilation with the patient in a lateral decubitus position. Port placement depends on tumor location and most surgeons recommend the use of three triangulated ports. Since most neurogenic tumors have a well-defined capsule, they can be resected by simple enucleation (68) with intercostal and paravertebral blood vessels being ligated, clipped, or coagulated with advanced bipolar cautery or ultrasonic energy devices (59,69). The pedicle of the tumor, which is usually the intercostal nerve, is then divided on both sides of the tumor. Depending on tumor size and location, traction injuries to the spinal cord and spinal nerve root avulsion may occur. To prevent such injuries, it is recommended to divide the involved intercostal nerve and tumor pedicle early in the operation (57).
If the tumor extends into the intervertebral foramen but extension remains extradural, it can also be approached by VATS. These tumors must, however, be approached with great caution and it is critical to avoid any kind of traction on the foraminal component so as to avoid root avulsion, cord injury, and cerebrospinal fluid leak (67). If there are dense adhesions between tumor and surrounding structures or if the tumor is located in a narrow space such as the thoracic inlet, it may be safer to convert to an open thoracotomy rather than persist with VATS and run the risk of serious neurovascular injuries.
Relative contra-indications to VATS resection of neurogenic tumors include malignant and invasive tumors, larger tumors (larger than 5 cm), tumors located in a narrow space such as at the thoracic apex, and tumors extending beyond the confines of the chest such as below the diaphragm or in the neck.
Successful robotic resection of posterior mediastinal tumors has also been reported (70) but these procedures are complex and considerable controversy persists regarding technical issues.
Surgery for dumbbell tumors
A dumbbell tumor is a neurogenic tumor where the thoracic component extends through the intervertebral foramen into the spinal canal. The term “dumbbell” refers to the shape of the tumor where its intrathoracic segment is connected to the intraspinal component by a narrow segment of tumor giving it a dumbbell configuration. This type of extension occurs in approximately 8–10% of neurogenic tumors, and about 60% of patients with such an involvement will have neurological signs and symptoms related to the compression of the intercostal nerve or spinal cord.
Although the surgical approach to dumbbell tumors is somewhat controversial, most surgeons recommend the use of a single-stage combined thoracic and neurosurgical open thoracotomy approach as advocated by the Boston Massachusetts General Hospital thoracic surgical group (71,72). If the intraspinal tumor is extensive, a posterior laminectomy is also required. A thoracoscopic approach for the resection of selected cases of dumbbell tumors has been reported (59,73,74) but is not generally recommended.
Conclusions
True mediastinal tumors and masses are lesions arising from structures normally located in the mediastinum or from structures that are transiting through it. In order to provide optimal surgical care, one should always establish a clear diagnosis and then fully investigate local extension before proceeding with surgery. In contemporary thoracic surgery, failure to do so should be considered negligent.
Enthusiasm for approaching these lesions through thoracoscopic or other minimally-invasive techniques began in the early 1990s when it became apparent that such techniques were both feasible and safe and that perioperative results compared favorably with those of open thoracotomy. Short and mid-term follow-up studies have also shown similar oncologic outcomes in terms of disease-free survival and incidence of local recurrences. Because of such results, VATS should now be considered as the “Standard of Care” for the treatment of selected mediastinal tumors and masses.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gray H. Gray’s Anatomy. The Anatomical Basis of Medicine and Surgery. 38th edition. New York: Churchill Livingstone, 1995.
- Liu W, Deslauriers J. Mediastinal divisions and compartments. Thorac Surg Clin 2011;21:183-90. viii. [Crossref] [PubMed]
- Sugarbaker DJ. Thoracoscopy in the management of anterior mediastinal masses. Ann Thorac Surg 1993;56:653-6. [Crossref] [PubMed]
- Detterbeck FC. Clinical value of the WHO classification system of thymoma. Ann Thorac Surg 2006;81:2328-34. [Crossref] [PubMed]
- Blumberg D, Burt ME, Bains MS, et al. Thymic carcinoma: current staging does not predict prognosis. J Thorac Cardiovasc Surg 1998;115:303-8; discussion 308-9. [Crossref] [PubMed]
- Chung DA. Thymic carcinoma--analysis of nineteen clinicopathological studies. Thorac Cardiovasc Surg 2000;48:114-9. [Crossref] [PubMed]
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Fang W, Chen W, Chen G, et al. Surgical management of thymic epithelial tumors: a retrospective review of 204 cases. Ann Thorac Surg 2005;80:2002-7. [Crossref] [PubMed]
- Wright CD, Wain JC, Wong DR, et al. Predictors of recurrence in thymic tumors: importance of invasion, World Health Organization histology, and size. J Thorac Cardiovasc Surg 2005;130:1413-21. [Crossref] [PubMed]
- Venuta F, Rendina EA, Pescarmona EO, et al. Multimodality treatment of thymoma: a prospective study. Ann Thorac Surg 1997;64:1585-91; discussion 1591-2. [Crossref] [PubMed]
- Nakahara K, Ohno K, Hashimoto J, et al. Thymoma: results with complete resection and adjuvant postoperative irradiation in 141 consecutive patients. J Thorac Cardiovasc Surg 1988;95:1041-7. [PubMed]
- Regnard JF, Magdeleinat P, Dromer C, et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg 1996;112:376-84. [Crossref] [PubMed]
- Jurado J, Javidfar J, Newmark A, et al. Minimally invasive thymectomy and open thymectomy: outcome analysis of 263 patients. Ann Thorac Surg 2012;94:974-81; discussion 981-2. [Crossref] [PubMed]
- Sakamaki Y, Oda T, Kanazawa G, et al. Intermediate-term oncologic outcomes after video-assisted thoracoscopic thymectomy for early-stage thymoma. J Thorac Cardiovasc Surg 2014;148:1230-1237.e1. [Crossref] [PubMed]
- Ye B, Tantai JC, Ge XX, et al. Surgical techniques for early-stage thymoma: video-assisted thoracoscopic thymectomy versus transsternal thymectomy. J Thorac Cardiovasc Surg 2014;147:1599-603. [Crossref] [PubMed]
- Pennathur A, Qureshi I, Schuchert MJ, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011;141:694-701. [Crossref] [PubMed]
- Toker A. Standardized definitions and policies of minimally invasive thymoma resection. Ann Cardiothorac Surg 2015;4:535-9. [PubMed]
- Odaka M, Akiba T, Yabe M, et al. Unilateral thoracoscopic subtotal thymectomy for the treatment of stage I and II thymoma. Eur J Cardiothorac Surg 2010;37:824-6. [Crossref] [PubMed]
- Port JL, Ginsberg RJ. Surgery for thymoma. Chest Surg Clin N Am 2001;11:421-37. [PubMed]
- Weksler B, Pennathur A, Sullivan JL, et al. Resection of thymoma should include nodal sampling. J Thorac Cardiovasc Surg 2015;149:737-42. [Crossref] [PubMed]
- Suda T, Hachimaru A, Tochii D, et al. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results†. Eur J Cardiothorac Surg. 2016;49 Suppl 1:i54-8. [PubMed]
- Suda T, Kaneda S, Hachimaru A, et al. Thymectomy via a subxiphoid approach: single-port and robot-assisted. J Thorac Dis 2016;8:S265-71. [PubMed]
- Suda T, Kaneda S, Hachimaru A, et al. Thymectomy via a subxiphoid approach: single-port and robot-assisted. J Thorac Dis 2016;8:S265-71. [PubMed]
- Li Y, Wang J. Left-sided approach video-assisted thymectomy for the treatment of thymic diseases. World J Surg Oncol 2014;12:398. [Crossref] [PubMed]
- Ohta M, Hirabayasi H, Okumura M, et al. Thoracoscopic thymectomy using anterior chest wall lifting method. Ann Thorac Surg 2003;76:1310-1. [Crossref] [PubMed]
- Fiorelli A, Mazzella A, Cascone R, et al. Bilateral thoracoscopic extended thymectomy versus sternotomy. Asian Cardiovasc Thorac Ann 2016;24:555-61. [Crossref] [PubMed]
- Wu CY, Heish MJ, Wu CF. Single port VATS mediastinal tumor resection: Taiwan experience. Ann Cardiothorac Surg 2016;5:107-11. [Crossref] [PubMed]
- Takeo S, Sakada T, Yano T. Video-assisted extended thymectomy in patients with thymoma by lifting the sternum. Ann Thorac Surg 2001;71:1721-3. [Crossref] [PubMed]
- Savitt MA, Gao G, Furnary AP, et al. Application of robotic-assisted techniques to the surgical evaluation and treatment of the anterior mediastinum. Ann Thorac Surg 2005;79:450-5; discussion 455. [Crossref] [PubMed]
- Ye B, Tantai JC, Li W, et al. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery in the surgical treatment of Masaoka stage I thymoma. World J Surg Oncol 2013;11:157. [Crossref] [PubMed]
- Marulli G, Rea F, Melfi F, et al. Robot-aided thoracoscopic thymectomy for early-stage thymoma: a multicenter European study. J Thorac Cardiovasc Surg 2012;144:1125-30. [Crossref] [PubMed]
- Schneiter D, Tomaszek S, Kestenholz P, et al. Minimally invasive resection of thymomas with the da Vinci® Surgical System. Eur J Cardiothorac Surg 2013;43:288-92. [Crossref] [PubMed]
- Lucchi M, Ambrogi MC, Duranti L, et al. Advanced stage thymomas and thymic carcinomas: results of multimodality treatments. Ann Thorac Surg 2005;79:1840-4. [Crossref] [PubMed]
- Daliakopoulos SI, Chatzoulis G, Lampridis S, et al. Gamma probe-assisted excision of an ectopic parathyroid adenoma located within the thymus: case report and review of the literature. J Cardiothorac Surg 2014;9:62. [Crossref] [PubMed]
- Said SM, Cassivi SD, Allen MS, et al. Minimally invasive resection for mediastinal ectopic parathyroid glands. Ann Thorac Surg 2013;96:1229-33. [Crossref] [PubMed]
- Wei B, Inabnet W, Lee JA, et al. Optimizing the minimally invasive approach to mediastinal parathyroid adenomas. Ann Thorac Surg 2011;92:1012-7. [Crossref] [PubMed]
- Kim YS, Kim J, Shin S. Thoracoscopic removal of ectopic mediastinal parathyroid adenoma. Korean J Thorac Cardiovasc Surg 2014;47:317-9. [Crossref] [PubMed]
- Onoda N, Ishikawa T, Nishiyama N, et al. Focused approach to ectopic mediastinal parathyroid surgery assisted by radio-guided navigation. Surg Today 2014;44:533-9. [Crossref] [PubMed]
- Amer K, Khan AZ, Rew D, et al. Video assisted thoracoscopic excision of mediastinal ectopic parathyroid adenomas: a UK regional experience. Ann Cardiothorac Surg 2015;4:527-34. [PubMed]
- Adachi Y, Nakamura H, Taniguchi Y, et al. Thoracoscopic resection with intraoperative use of methylene blue to localize mediastinal parathyroid adenomas. Gen Thorac Cardiovasc Surg 2012;60:168-70. [Crossref] [PubMed]
- Lewis BD, Hurt RD, Payne WS, et al. Benign teratomas of the mediastinum. J Thorac Cardiovasc Surg 1983;86:727-31. [PubMed]
- Sasaka K, Kurihara Y, Nakajima Y, et al. Spontaneous rupture: a complication of benign mature teratomas of the mediastinum. AJR Am J Roentgenol 1998;170:323-8. [Crossref] [PubMed]
- Kesler KA, Rieger KM, Ganjoo KN, et al. Primary mediastinal nonseminomatous germ cell tumors: the influence of postchemotherapy pathology on long-term survival after surgery. J Thorac Cardiovasc Surg 1999;118:692-700. [Crossref] [PubMed]
- St-Georges R, Deslauriers J, Duranceau A, et al. Clinical spectrum of bronchogenic cysts of the mediastinum and lung in the adult. Ann Thorac Surg 1991;52:6-13. [Crossref] [PubMed]
- Weber T, Roth TC, Beshay M, et al. Video-assisted thoracoscopic surgery of mediastinal bronchogenic cysts in adults: a single-center experience. Ann Thorac Surg 2004;78:987-91. [Crossref] [PubMed]
- Jung HS, Kim DK, Lee GD, et al. Video-assisted thoracic surgery for bronchogenic cysts: is this the surgical approach of choice? Interact Cardiovasc Thorac Surg 2014;19:824-9. [Crossref] [PubMed]
- De Giacomo T, Diso D, Anile M, et al. Thoracoscopic resection of mediastinal bronchogenic cysts in adults. Eur J Cardiothorac Surg 2009;36:357-9. [Crossref] [PubMed]
- Martinod E, Pons F, Azorin J, et al. Thoracoscopic excision of mediastinal bronchogenic cysts: results in 20 cases. Ann Thorac Surg 2000;69:1525-8. [Crossref] [PubMed]
- Panchanatheeswaran K, Dutta R, Singh KI, et al. Eleven-year experience in thoracoscopic excision of bronchogenic cyst. Asian Cardiovasc Thorac Ann 2012;20:570-4. [Crossref] [PubMed]
- Mouroux J, Benchimol D, Bernard JL, et al. Exérèse d’un Kyste Bronchogénique par Video-Thoracoscopie. Presse Med 1991;20:1768-9. [PubMed]
- Ginsberg RJ, Atkins RW, Paulson DL. A bronchogenic cyst successfully treated by mediastinoscopy. Ann Thorac Surg 1972;13:266-8. [Crossref] [PubMed]
- Hazelrigg SR, Landreneau RJ, Mack MJ, et al. Thoracoscopic resection of mediastinal cysts. Ann Thorac Surg 1993;56:659-60. [Crossref] [PubMed]
- Borges AC, Gellert K, Dietel M, et al. Acute right-sided heart failure due to hemorrhage into a pericardial cyst. Ann Thorac Surg 1997;63:845-7. [Crossref] [PubMed]
- Satur CM, Hsin MK, Dussek JE. Giant pericardial cysts. Ann Thorac Surg 1996;61:208-10. [Crossref] [PubMed]
- Deslauriers J. Diagnosis and management of thoracic neurogenic tumours. Can J Surg 1992;35:470. [PubMed]
- Takeda S, Miyoshi S, Minami M, et al. Intrathoracic neurogenic tumors--50 years' experience in a Japanese institution. Eur J Cardiothorac Surg 2004;26:807-12. [Crossref] [PubMed]
- Shields TW, Reynolds M. Neurogenic tumors of the thorax. Surg Clin North Am 1988;68:645-68. [Crossref] [PubMed]
- Barrenechea IJ, Fukumoto R, Lesser JB, et al. Endoscopic resection of thoracic paravertebral and dumbbell tumors. Neurosurgery 2006;59:1195-201; discussion 1201-2. [Crossref] [PubMed]
- Ricci C, Rendina EA, Venuta F, et al. Diagnostic imaging and surgical treatment of dumbbell tumors of the mediastinum. Ann Thorac Surg 1990;50:586-9. [Crossref] [PubMed]
- Yang C, Zhao D, Zhou X, et al. A comparative study of video-assisted thoracoscopic resection versus thoracotomy for neurogenic tumours arising at the thoracic apex. Interact Cardiovasc Thorac Surg 2015;20:35-9. [Crossref] [PubMed]
- Ribet ME, Cardot GR. Neurogenic tumors of the thorax. Ann Thorac Surg 1994;58:1091-5. [Crossref] [PubMed]
- Landreneau RJ, Dowling RD, Ferson PF. Thoracoscopic resection of a posterior mediastinal neurogenic tumor. Chest 1992;102:1288-90. [Crossref] [PubMed]
- Riquet M, Mouroux J, Pons F, et al. Videothoracoscopic excision of thoracic neurogenic tumors. Ann Thorac Surg 1995;60:943-6. [Crossref] [PubMed]
- Bousamra M 2nd, Haasler GB, Patterson GA, et al. A comparative study of thoracoscopic vs open removal of benign neurogenic mediastinal tumors. Chest 1996;109:1461-5. [Crossref] [PubMed]
- Liu HP, Yim AP, Wan J, et al. Thoracoscopic removal of intrathoracic neurogenic tumors: a combined Chinese experience. Ann Surg 2000;232:187-90. [Crossref] [PubMed]
- Pons F, Lang-Lazdunski L, Bonnet PM, et al. Videothoracoscopic resection of neurogenic tumors of the superior sulcus using the harmonic scalpel. Ann Thorac Surg 2003;75:602-4. [Crossref] [PubMed]
- Endo S, Murayama F, Otani S, et al. Alternative surgical approaches for apical neurinomas: a thoracoscopic approach. Ann Thorac Surg 2005;80:295-8. [Crossref] [PubMed]
- Ponce FA, Killory BD, Wait SD, et al. Endoscopic resection of intrathoracic tumors: experience with and long-term results for 26 patients. J Neurosurg Spine 2011;14:377-81. [Crossref] [PubMed]
- Li Y, Wang J. Experience of Video-Assisted Thoracoscopic Resection for Posterior Mediastinal Neurogenic Tumors: A Retrospective Analysis of 58 Patients. ANZ J Surg 2013;83:664-8. [Crossref] [PubMed]
- Fraga JC, Rothenberg S, Kiely E, et al. Video-assisted thoracic surgery resection for pediatric mediastinal neurogenic tumors. J Pediatr Surg 2012;47:1349-53. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Operative techniques in robotic thoracic surgery for inferior or posterior mediastinal pathology. J Thorac Cardiovasc Surg 2012;143:1138-43. [Crossref] [PubMed]
- Shadmehr MB, Gaissert HA, Wain JC, et al. The surgical approach to "dumbbell tumors" of the mediastinum. Ann Thorac Surg 2003;76:1650-4. [Crossref] [PubMed]
- Heltzer JM, Krasna MJ, Aldrich F, et al. Thoracoscopic excision of a posterior mediastinal "dumbbell" tumor using a combined approach. Ann Thorac Surg 1995;60:431-3. [Crossref] [PubMed]
- Tsunezuka Y, Sato H. Video-assisted thoracoscopy in single-stage resection of a para-aortic posterior mediastinal dumbbell tumor. Thorac Cardiovasc Surg 1998;46:47-9. [Crossref] [PubMed]
Cite this article as: Rakovich G, Deslauriers J. Video-assisted and minimally-invasive open chest surgery for the treatment of mediastinal tumors and masses. J Vis Surg 2017;3:25.


