Anatomical video-assisted thoracoscopic surgery segmentectomies based on the three-dimensional reformation images
Introduction
Video-assisted thoracoscopic surgery (VATS) is one of two new technologies for nearly 30 years in the field of thoracic surgery. Due to its outstanding advantages (light postoperative pain, fewer complications, faster recovery, beautiful appearance, etc.) and equal to or even better than the traditional thoracotomy in short and long term results, which is a powerful attraction for doctors and patients (1). The major procedures included lobectomy, wedge resection, and segmentectomy. For the central lesions, they were closely associated with bronchus and pulmonary artery (PA). Wedge resection is difficult and has more risk of hemorrhage. For old patients with restricted lung function who cannot tolerate lobotomy, the anatomic lung segmentectomy attracted everyone's attention and has become a hot research area in recent years (2).
Patient selection and clinical summary
In this study, four anatomical VATS segmentectomy videos clips were shared. They are right upper lobe posterior segmentectomy, left upper lobe anterior segmentectomy, left lower lobe superior segmentectomy, and left lingular sparing upper lobectomy.
Case No. 1: a 49-year-old gentleman was admitted because of a nodule was found in the right upper lobe after chest CT examination. He received anti-TB treatment for one month. However, the size of nodule increased from 2.0 cm × 2.0 cm to 2.5 cm × 2.5 cm, and the signs of pleural retraction can be found (Figure 1). This patient was transferred to thoracic department due to suspicion of lung cancer. Pulmonary function test: FEV1: 1.84 L (64%), nothing special were found through the bronchoscope, PET-CT examination found no signs of metastasis, blood tumor markers carcinoembryonic antigen (CEA): 6.7 μg/L (reference value <5 μg/L). There was no preoperative pathology, the lesion is deep, close with bronchus and pulmonary vessels. Wedge resection is not suitable. The patient received right upper lobe posterior segmentectomy to confirm the pathology. Incision: 4 cm in the 4th intercostal space on the right anterior axillary line; camera port: 1.5 cm in 8th intercostal on right middle axillary line. Operation took 2 hours, blood loss was 100 mL. No postoperative complication happened; the patient was discharged on the 7th day postoperatively. Final pathology was confirmed with adenocarcinoma. PTNM: T1cN0M0 stage IA3 (Figure 2).
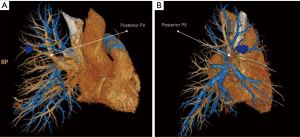

Case No. 2: a 67-year-old male was admitted due to “lower extremity pain for more than one year”. Chest CT: left upper lobe mass with the size of 3.0 cm × 3.5 cm, pulmonary function tests: FEV1 3.1 L (94%). He received VATS left upper lobe anterior segmentectomy. Operation hole: 4 cm incision in the left 4th intercostal on the anterior axillary line; observation hole: 1.5 cm incision at the 7th intercostal space on the left mid-axillary line. The operation took 5 hours, blood loss was 400 mL due to serious chest cavity adhesion. Postoperative complications were not observed, and he discharged on the 6th day postoperatively. Pathology: bronchiolitis obliterans organizing pneumonia, BOOP (Figure 3).

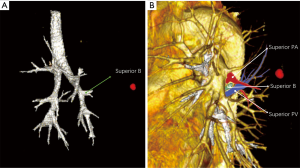
Case No. 3: a 71-year-old female was admitted due to a ground glass opacity (GGO) was found after chest CT screening. The size of the GGO (1.5 cm × 1.2 cm) located in the superior segment of the left lower lobe (Figure 4). Pulmonary function tests: FEV1 1.8 L (71%). The patient received thoracoscopic left lower lobe superior segmentectomy. Operative hole: 4 cm incision in the 4th intercostal space on the left anterior axillary line; camera port: 1.5 cm incision in the 8th intercostal space on the left middle axillary line; assistant port: 1.5 cm incision in the 9th intercostal space on the left inferior angle line of scapula. Operation time took 2 hours, blood loss was 100 mL. There were no postoperative complications. She was discharged on the 7th day postoperatively. Pathology: invasive adenocarcinoma, lepidic growth predominant (90%), T1bN0M0, stage IA2. No signs of recurrence or metastasis were found in 2 years’ follow-up (Figure 5).

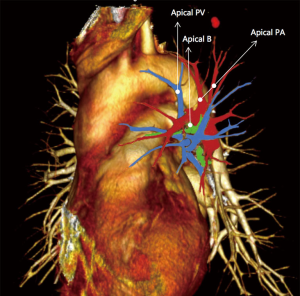
Case No. 4: a 73-year-old woman was admitted for founding a GGO during the screening test (1.0 cm × 1.0 cm) (Figure 6). Her pulmonary function result was FEV1: 1.51 L (54.7% predicted). She was a non-smoker, with negative bronchoscopy findings. There were no positive past medical history and co-morbidities. The first 1.5 cm incision was selected in the eighth intercostal space in the midaxillary line, and was used for the camera (30 degree 10 mm high definition video thoracoscope). A 4-cm long incision was made in the fourth intercostal space in the preaxillary line. A third 1.5 cm incision was performed in the ninth intercostal space in the postaxillary line for assistant. Final pathology was confirmed with adenocarcinoma (ancinar component dominant), T1N0M0 stage IA. Chest X-ray showed left lingular segment and left lower lobe reexpanded. Analgesia, antibiotics are used for 2 days postoperatively. There were no complications and the patient was discharged 6 days postoperatively (Figure 7).

Pre-operative assessment
Standard lobectomy is associated with respiratory failure for elder patients with compromised pulmonary function. Segmentectomy was an alternative. Patients’ lesions were evaluated precisely based on the three-dimensional reformatted images. And they were selected to be candidates for segmentectomy. Preoperative workup included clinical history, physical examination, chest computed tomography (CT) scan with intravenous contrast no more than 1 month before resection, pulmonary function test, blood gas analysis, cardiac evaluation, bronchoscopy and basic examinations as usual. Abdominal B-ultrasound, cerebral magnetic resonance imaging (MRI) and isotopic bone scanning were examinations to exclude metastatic disease. FDG-PET (fluorodeoxyglucose-position emission tomographies) was employed to exclude N2 disease. Three-dimensional reformation images were obtained from the IntelliSpace Portal Work Station. Smokers were stopped for at least 2 weeks before operation.
Anaesthesia and positioning
All patients received general anesthesia with double-lumen endotracheal intubation and healthy lung ventilation. Lateral decubitus position was chosen. Their arms extended to 90° and the elbows flexed to 90°. And the operative table was flexed to maximize the intercostal space and drop the hip down to facilitate the camera’s position.
Operation technology
Thoracoscopic segmentectomy of lung is similar to lobectomy under VATS (2). Pulmonary ligament and the entire left hilum should be mobilized with combination of sharp and blunt dissection. Usually from the base of pulmonary blood vessels to the distal dissection, clear the supply of blood vessels of lung segments, staple pulmonary vein (PV), and then staple pulmonary arteries and bronchus according to the different lung segments. Or staple lung segment bronchus firstly and staple PA secondly, finally cut lung tissues along the intersegmental plane. The vein of lung segment and lung tissue can be cut together in some situations. Mediastinal lymph nodes dissection could be completed by electronic hook or harmonic scalpel. Injury of the adjacent structures like apical PA, phrenic and recurrent laryngeal nerves must be avoided during this step.
Take the left lingular sparing upper lobectomy for example; the superior PV has usually three major tributaries. The superior branch drains the apicoposterior segments and frequently blocks the access to the apicoposterior arteries. The middle branch drains the anterior segment, and the lowermost branch drains the lingula. The lingular vein must be preserved. The apicoposterior and anterior segment vein was transected with a vascular stapler. The upper lobe bronchus splits immediately into the lingular bronchus and a common stem. All these segmental bronchi have short course and a calcified lymph node located between the apicoposterior PA and apicoposterior bronchus. These situations make the dissection and identification very difficult. Following many failure attempts of trying take the calcified lymph node out. Staple the left apicoposterior PA together with the apicoposterior bronchi is completed. And left upper division (S1+2 and S3) was taken out after stapling lung tissue above the level of lingular segment with a 60 mm green linear stapler. Mediastinal lymph nodes of level 9, 7, 4L and 5 were cleared afterwards. The specimen was removed with a bag. Bronchial stump was confirmed without air leakage by water test. Staple the left apicoposterior PA together with the apicoposterior bronchi is a safe and feasible way when facing the difficult dissection of the calcified lymph nodes during segmentectomy. However, control of the left main PA is a recommendation before fire the stapler.
Discussion
In the past 80 years, lung disease spectrum has changed a lot, the incidence of lung adenocarcinoma increased year by year (7). Early lung adenocarcinoma of lung ground glass (GGO) becomes more common.
It seems that the approach of thoracoscopic segmentectomies are technically challenging. However, the operation time and the result of these procedures are satisfied with the help of the three-dimensional reformation image. VATS segmentectomies is safe and feasible for early stage lung adenocarcinoma, especially for elder patients with compromised pulmonary function. The thoracoscopic criteria should include hilar division of bronchovascular elements, adequate clearance of intersegmental lymph nodes and tumor-free margins.
Specific indications for lung segment resection as follows (8-11): (I) IA in peripheral lung shadows, maximum diameter as follows especially for elder patients with compromised pulmonary function 2 cm from the cutting edge); (II) tumor solid component ratio maximum diameter as follows esive adenocarcinoma, Calculation method is the maximum diameter mediastinal window tumor solid components/the maximum diameter of lung window tumor; (III) high-resolution CT showed no swelling lymph nodes in mediastinal and pulmonary hilar and (or) FDG-PET show no tracer concentration in lymph node of mediastinal and pulmonary hilar; (IV) intraoperative frozen pathology showed lesions are atypical adenomatous hyperplasia, lung adenocarcinoma in situ, tiny invasive adenocarcinoma and adherent growth-oriented invasive adenocarcinoma, and N1, N2 lymph nodes and the cutting edge are negative, also can be used for cytology pathology after washing the edge of Endo-GIA stapler to exclude the possibility of positive margin; (V) involving the different lobe nodules require surgery over the same period; (VI) poor cardiopulmonary function, first second forced expiratory volume percentage of predicted value <50%; (VII) age >75 years, limited lung function compensation after surgery; (VIII) more complications, cannot do lobectomy; (IX) the patient’s own choice; (X) as a surgery method for cancer recurrent in lobectomy.
CT three-dimensional reformation could exactly show the small pulmonary nodules’ location and facilitate VATS segmentectomies. Unnecessary explore time for localizing small pulmonary nodules during the operation could be avoid. The conversion rate from VATS to the open procedure could be reduced. Software and training for reconstruction is not difficult and can be applied in majority medical centers (12,13).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Swanson SJ. Video-assisted thoracic surgery segmentectomy: the future of surgery for lung cancer? Ann Thorac Surg 2010;89:S2096-7. [Crossref] [PubMed]
- Yang CF, D'Amico TA. Thoracoscopic segmentectomy for lung cancer. Ann Thorac Surg 2012;94:668-81. [Crossref] [PubMed]
- Ma Q, Bao T, Liu D, et al. Right upper lobe posterior segmentectomy. Asvide 2017;4:041. Available online: http://www.asvide.com/articles/1347
- Ma Q, Bao T, Liu D, et al. Left upper lobe anterior segmentectomy. Asvide 2017;4:042. Available online: http://www.asvide.com/articles/1348
- Ma Q, Bao T, Liu D, et al. Left lower lobe superior segmentectomy. Asvide 2017;4:043. Available online: http://www.asvide.com/articles/1349
- Ma Q, Bao T, Liu D, et al. Left lingular sparing upper lobectomy. Asvide 2017;4:044. Available online: http://www.asvide.com/articles/1350
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Zhong C, Fang W, Mao T, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy for small-sized stage IA lung cancer. Ann Thorac Surg 2012;94:362-7. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg 2013;146:358-64. [Crossref] [PubMed]
- Yendamuri S, Sharma R, Demmy M, et al. Temporal trends in outcomes following sublobar and lobar resections for small (small (llowing sublobar and lobars--a Surveillance Epidemiology End Results database analysis. J Surg Res 2013;183:27-32. [Crossref] [PubMed]
- Houck WV, Fuller CB, McKenna RJ Jr. Video-assisted thoracic surgery upper lobe trisegmentectomy for early-stage left apical lung cancer. Ann Thorac Surg 2004;78:1858-60. [Crossref] [PubMed]
- Limmer S, Dicken V, Kujath P, et al. Three-dimensional reconstruction of central lung tumors based on CT data. Chirurg 2010;81:833-40. [Crossref] [PubMed]
- Ikeda N, Yoshimura A, Hagiwara M, et al. Three dimensional computed tomography lung modeling is useful in simulation and navigation of lung cancer surgery. Ann Thorac Cardiovasc Surg 2013;19:1-5. [Crossref] [PubMed]
Cite this article as: Ma Q, Bao T, Zhang H, Liang C, Liu D. Anatomical video-assisted thoracoscopic surgery segmentectomies based on the three-dimensional reformation images. J Vis Surg 2017;3:21.


