Robot-assisted surgery in complex treatment of the pulmonary tuberculosis
Introduction
Currently, thoracotomy is the standard access to perform lung resections for tuberculosis, despite of the widespread use of the video-assisted thoracoscopic surgery (VATS) technique since 1990 and robot-assisted thoracoscopic surgery (RATS) technique since 2004. VATS therapeutic lung resection for tuberculosis is performed only in a few thoracic centers today. First robot-assisted lobectomy for pulmonary tuberculomas was performed in St. Petersburg State Research Institute of Phthisiopulmonology in 2013 (1). Factors, such as dense adhesions in the pleural cavity and hilar structures, limit the using of minimally invasive approach for patients with pulmonary tuberculosis (2). The purpose of this publication was to show the tips and tricks of robotic operations for pulmonary tuberculosis.
Selection of patients
Elective indications for robotic surgery are the same as for VATS. The duration and quality of the treatment before surgery are important to avoid relapses of the disease. Especially it is crucial in the cases of multi-drug resistant (MDR) and extensively drug-resistant (XDR) tuberculosis. Principles of operations in pulmonary tuberculosis are published in the World Health Organization guidelines, 2014. Thereby, it is necessary to observe the conditions for the surgery: localized forms of tuberculosis, disease-free lung tissue around the resection margins, and acceptable surgical risk of pulmonary resection. The main elective indications for surgery in tuberculosis are persistent cavitary tuberculosis after four to 6 months of supervised anti-tuberculosis chemotherapy, failure of anti-tuberculosis chemotherapy in MDR and XDR tuberculosis-cases, complications and sequelae of the tuberculosis process (3).
The key point of the patient’s selection is prediction of pleural adhesions in tuberculosis-cases. This is important when using the DaVinci Si surgical system, as long as it causes features of surgical access.
Robot-assisted lobectomy: technique of operation
Different authors proposed VATS based approach (3–4 ports) and total port approach (5 ports) for RATS lobectomy (4,5). Location of instrumental trocars and assistant port were also different. There is no unified technique of the RATS lobectomy today. The use of both methods depends on the surgeon’s preference. Some clinics are promoting operation technique with passive assistant. It is 4-arms method usually. We believe that it is sufficient to use three robotic ports performing lobectomy if the assistant is actively working. It gives the operation more dynamic.
We use modified technique of Mark R. Dylewski (4). There are two reasons for modifying the surgical technique: the characteristics of a robotic surgical system Si (it is not possible to divide the adhesions below trocars) and high incidence of pleural adhesions in tuberculosis.
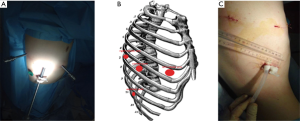
The patient is flexed in the lateral decubitus position. The points of trocars insertion are (Figure 1): 1st incision (camera port): 6–7th intercostal space (ICS) at the posterior axillary line, 2nd incision (assistant port): 9–10th ICS, 3rd incision (instrumental port): 5–6th ICS at the anterior axillary line, 4th incision (instrumental port): 7–8th ICS at the scapular line.
The location of assistant port depends on the type of lobectomy. Insertion point of trocar is placed posteriorly for upper lobectomy and anteriorly for lower lobectomy. These differences are depending on the angle needed for introducing of the endo-stapler for dividing pulmonary vein.
One of the difficulties of tuberculosis surgery is the division of pleural adhesions. It is not difficult for robotic surgery, if the adhesions are located at the apex of pleural cavity. However, adhesion under diaphragm is sometimes unavailable for robotic tools (in DaVinci Si version). The first decision of this problem is the lowest port’s placement. This feature allows to dividing adhesions up to the port’s insertion line (Figure 2).

On the other hand, an assistant can divide adhesions into the lower parts of the pleural cavity using VATS techniques (Figure 3).

Due to limitations of movements of robotic tools above the diaphragm, once we used repositioning of patient cart of robotic system. We called this method as a re-docking procedure. The standard position of the patient cart is at angle of 15 degrees to the patient’s head. The target point for surgical system localized in the apex of pleural cavity in this position. To move the target point above the diaphragm we placed the patient cart at an angle of 175–185 degrees to the patient’s head. Camera and assistant ports remain in their places. Left and right instrumental ports were changed between themselves. Robot-assisted thoracoscopic division of adhesions over the diaphragm was more comfortable in this position of the patient cart. After pneumolysis patient cart moved to the original position. This procedure increased the console operative time not more than for 20 minutes.
Steps of robot-assisted lobectomy follow the standard surgical steps of open lobectomy (Figure 4). The surgeon performs division of hilar structures using a robotic surgical system. An assistant performs dividing and closing of the hilar structures and lung tissue, removes of the specimen and place the drain under the surgeon’s control.
Features of different types of robot-assisted lobectomy
There are many guidelines for the technique of minimally invasive lobectomy today. Nevertheless, there are many different tricks, which make it easier to perform the procedures in every major center. Here are some of them.
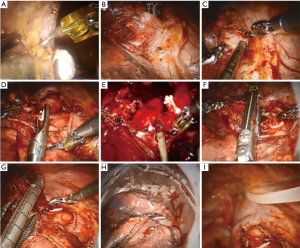
Right upper lobectomy: we always use the anterior approach for isolation of the hilar structures (Figure 5). At the same time, we usually divide the posterior segment’s artery after bronchial step. This is important, since we have had a few cases, when arterial clips were sliding during the isolation bronchus.

Left upper lobectomy: it is inconvenient and much longer to begin fissureless lobectomy with isolation of hilar structures in the interlobar fissure, especially due to gravity of the left upper lobe. Anterior approach provides a good control of the vein and bronchus. Firstly, we identified and divide of the upper pulmonary vein and A1–3 segmental artery. A feature of a bronchial step is carefully isolation of the posterior bronchial wall due to the location of the pulmonary artery (Figure 6). There is an excellent visualization of segmental arteries after closing of the bronchus. These arteries could be isolated and clipped separately. However, the dividing of interlobar space is possible with segmental arteries of the upper lobe during this step.

Lower lobectomies. A feature of these operations is the lower port placement than the upper lobectomy. Careful dissection of the oblique interlobar fissure is required for visualization and isolation A6 and basal arteries. In addition, we use stapler with a curved tip at the distal end of the anvil providing enhanced visibility and maneuverability (Figure 7).

Right middle lobectomy. This is a rare lobectomy for tuberculosis. This procedure is technically simple in cases with good interlobar fissure. The first step is to visualize and isolation of middle lobe vein. Preliminary separation of lung tissue between segments S5 and S7 considerably facilitates of the isolation of hilar structures (Figure 8). Isolation of A4–5 artery in the interlobar fissure is the good decision for cases with dense adhesions in the hilum. Nevertheless, in cases with the absence of interlobar fissures we also use anterior approach and consistently isolate and divide the vein, bronchus and artery.

Postoperative period
Results of surgery depend on the quality of post-operative treatment of patients with tuberculosis. Treatment protocol of our thoracic center was published in the World Health Organization guideline, and includes proper analgesia, respiratory exercises, daily chest X-rays for the first three days; early, as possible, and removal of chest tubes (3). A key factor of the postoperative treatment is the early prescription of anti- tuberculosis chemotherapy. The bacteriological examination of the specimen is also necessary to determine the degree of the mycobacterium tuberculosis (MTB) drug resistance (3).
Culture-positive patients at the time of surgery must continue the treatment during 6, 18 and 24 months after culture conversion for susceptible, MDR and XDR tuberculosis respectively. These periods might be shorter if the patient has culture-negative sputum at the time of surgery (4 and 8 months for susceptible and MDR and XDR tuberculosis respectively) (3).
Personal experience of RATS lobectomy
Since May 2013, 53 patients with pulmonary tuberculosis were selected for robot-assisted lobectomy (da Vinci Si Robotic System). History of the disease was 28+14 months. Rate of patients with persistent cavitary tuberculosis was 89%. There were 35% of patients with positive sputum smears on MTB despite of the supervised anti-tuberculosis chemotherapy. Mean age of the patients was 38+14 years. The majority of patients were smoking at the time of surgery (18+15 pack/years). Cardiopulmonary function tests showed an adequate pulmonary reserve (forced expiratory volume in 1 second was 3.6+0.97 L). Mean Charlson comorbidity index was 1.3+1.9. All patients had no exacerbation of chronic diseases at the time of surgery.
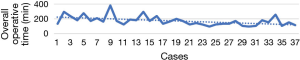
Most frequent surgery was the right upper lobectomy (37 patients/69%). Learning curve of this operation is shown in Figure 9.
Other types of lobectomy were performed with the same frequency (right lower lobectomy—five patients, left upper lobectomy—five patients, left lower lobectomy—four patients).
Forty-eight cases (90%) were associated with pleural adhesions. Total obliteration of pleural cavity was only in three cases (6%). Extrapleural mobilization of lung was performed in seven cases (13%) with subpleural location of the cavity.
There were two cases (4%) with conversion to open surgery. In one case, the procedure was converted to thoracotomy due to dense pleural adhesions in the pulmonary hilum. In another case conversion was accompanied with traction gap of the pulmonary artery between A2 and A6 during divided of interlobar fissure. Bleeding was stopped by pressure. After thoracotomy some stitches of the pulmonary artery was performed to final stop of the bleeding (blood loss was less than 150 mL).
Overall, operative time was 175+64 min and included docking time (17+6 min) and console operative time (109+62 min). Intraoperative blood loss was 82+95 mL (10–500 mL). Postoperative complications were registered by Ottawa Thoracic Morbidity & Mortality Classification System (12). There were 7 (13%) minor and 6 (11%) major complications. Mean duration of air leak was 3±1 postoperative day. Minor complications were mostly associated with small pneumothorax after removal of the chest tube, prolonged air leak, arrhythmia, pleuritis. Severe complications were acute gastrointestinal bleeding treated by endoscopy), hematoma of the right lobe of the liver (required laparotomy), exacerbation of chronic obstructive pulmonary disease (required bronchoscopy), prolonged air leak (required re-insertion of the chest tube) and pleuritis (treated by puncture). About 70% of complications and all serious complications (that require reoperation) were during the learning curve.
Mean pain level was three points in first postoperative day and two in fifth postoperative day by visual analogue scale. Analysis of removed lung specimens showed that mean degree of tuberculosis activity was 3±1 by B.M. Ariel score. There were positive smears of MTB in the 79% of specimens.
RATS lobectomy for two-sided pulmonary tuberculosis
Today, surgical treatment of patients with bilateral destructive pulmonary MDR tuberculosis is one of the controversial issues. This clinical case shows our first experience with consecutive robot-assisted thoracoscopic pulmonary lobectomies in combination with therapeutically treatment in such situation.
Male, 22 years old, was admitted in Chest center 24/11/2014. Tuberculosis was diagnosed with a planned X-ray examination in 2010. Initial diagnosis was tuberculosis of the right upper lobe, MTB (–). Primary course of treatment for tuberculosis lasted during 6 months with positivity. Patient was removed from the register.
Relapse of tuberculosis with MDR of MTB was registered in 2014. There was no positivity on the background of anti-tuberculosis treatment based on drug susceptibility test of MTB during 6 months. Nevertheless, there were persistent positive smears on MTB and bilateral destructive cavity in upper lobes of the both lungs. Failure of drug therapy was an indication for surgery.
At admission, the patient had complaints of dyspnea (Modified Medical Research Council Dyspnea Scale—two points). Examinations showed: positive sputum smears on MTB. Spirometry was consistent with moderate chronic obstructive pulmonary disease without exacerbation (Table 1). Perfusion scintigraphy showed severe disturbance of blood flow in the lateral section of the upper lobe of the right lung and the middle third of the upper lobe of the left lung. Computed tomography-scans before surgery presented on the Figure 10 (fibrous cavity in right upper lobe and thin-walled cavity in the left upper lobe). Charlson comorbidity index was one point.
Full table
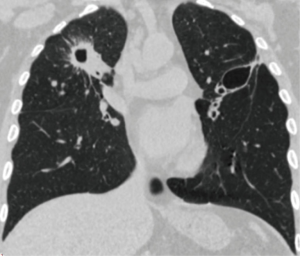
The first stage of treatment (15 December 2014) was installation of endobronchial valve in the left upper lobe bronchus. The procedure was performed without complications. Accordance to A. Levin and coauthors investigation (during 2008–2014 years) endobronchial valve treatment can significantly improve the effectiveness chemotherapy for MDR tuberculosis (13). We chose this method for the left side, because the chance of lobe collapse is higher, when thin wall of the cavity presence, than in cases of fibrous cavity.
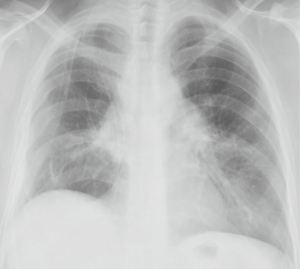
The second stage (15 January 2015) was robot-assisted thoracoscopic right upper lobectomy. Overall operation time was 155 min (console time was 120 minutes), blood loss was minimal. There were no postoperative complications (Figure 11). Chest tube was removed on postoperative day 4. Postoperatively, anti-tuberculosis treatment was continued according to drug susceptibility test of MTB.
Six months after the operation (17 August 2015) patient was readmitted to the chest center. There was conversion of the sputum smear on MTB. Nevertheless, polymerase chain reaction test on MTB was positive. Computed tomography scan showed thin-walled cavity in the left upper lobe without any dynamics. Spirometry parameters presented in the Table 1.
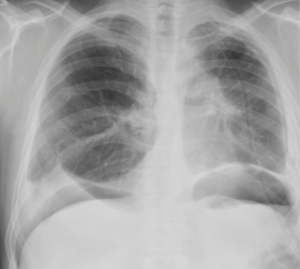
The third stage of treatment was robot-assisted thoracoscopic left upper lobectomy. Total obliteration of the pleural cavity was found during the installation of the first port. Separation of adhesions started with VATS approach. After that, operation continued with robotic system. Pneumolysis was performed in the intrapleural layer. Overall operation time was 280 min. (console time was 230 minutes), blood loss was 100 mL. Prolonged air leak was in the postoperative period. Artificial pneumoperitoneum was performed 2 times (Figure 12). Chest tubes removed on the postoperative day 14. Results of morphological and bacteriological examinations of the removed pulmonary lobes presented in the Table 2.
Full table
One year after the last operation patient had negative sputum smear on MTB. There were save of the dyspnea at the maintenance level (Modified Medical Research Council Dyspnea Scale—2 points), light sensitivity disorders in the field of postoperative scarring. Computed tomography of the chest showed no progression of the tuberculosis.
Conclusions
Robot-assisted lobectomy demonstrated efficacy and safety in standard lobectomy with pulmonary tuberculosis. Excellent results of robotic surgery allow performing this type of operations at patients with advanced pulmonary tuberculosis lesions. Several features, which were reviewed in this article, can help to perform the robot-assisted operations in the cases of extended adhesions.
Acknowledgements
None
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yablonskii P, Kudriashov G, Nuraliev S, et al. Perioperative outcomes of RATS pulmonary lobectomy for lung cancer and tuberculosis in a learning curve setting. European Respiratory Journal. 2015;46:PA2506. [Crossref]
- Yen YT, Wu MH, Lai WW, et al. The role of video-assisted thoracoscopic surgery in therapeutic lung resection for pulmonary tuberculosis. Ann Thorac Surg 2013;95:257-63. [Crossref] [PubMed]
- World Health Organization. The role of surgery in the treatment of pulmonary TB and multidrug-and extensively drug-resistant TB. Available online: http://www.euro.who.int/en/health-topics/communicable-diseases/tuberculosis/publications/2014/the-role-of-surgery-in-the-treatment-of-pulmonary-tb-and-multidrug-and-extensively-drug-resistant-tb
- Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg 2011;23:36-42. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Yablonskii P, Kudriashov G, Vasilev I, et al. Division of the pleural adhesion with robotic tools (over the diaphragm). Asvide 2017;4:017. Available online: http://www.asvide.com/articles/1323
- Yablonskii P, Kudriashov G, Vasilev I, et al. Division of the pleural adhesion through an assistant port (over the diaphragm). Asvide 2017;4:018. Available online: http://www.asvide.com/articles/1324
- Yablonskii P, Kudriashov G, Vasilev I, et al. Robot-assisted thoracoscopic surgery (RATS) right upper lobectomy. Asvide 2017;4:019. Available online: http://www.asvide.com/articles/1325
- Yablonskii P, Kudriashov G, Vasilev I, et al. Robot-assisted thoracoscopic surgery (RATS) left upper lobectomy. Asvide 2017;4:020. Available online: http://www.asvide.com/articles/1326
- Yablonskii P, Kudriashov G, Vasilev I, et al. Robot-assisted thoracoscopic surgery (RATS) right lower lobectomy. Asvide 2017;4:021. Available online: http://www.asvide.com/articles/1327
- Yablonskii P, Kudriashov G, Vasilev I, et al. Robot-assisted thoracoscopic surgery (RATS) right middle lobectomy. Asvide 2017;4:022. Available online: http://www.asvide.com/articles/1328
- Seely AJ, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg 2010;90:936-42; discussion 942. [Crossref] [PubMed]
- Levin A, Sklyuev S, Felker I, et al. Endobronchial valve treatment of destructive multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2016;20:1539-1545. [Crossref] [PubMed]
Cite this article as: Yablonskii P, Kudriashov G, Vasilev I, Avetisyan A, Sokolova O. Robot-assisted surgery in complex treatment of the pulmonary tuberculosis. J Vis Surg 2017;3:18.