Nonintubated video-assisted thoracic surgery lobectomy for lung cancer
Introduction
The development of video-assisted thoracic surgery (VATS) has revolutionized how surgeons treat thoracic diseases, demonstrating a paradigm shift toward an era of minimally invasive thoracic surgery (1). Currently, VATS lobectomy is performed worldwide to treat early-stage lung cancer, as it has equivalent oncological outcomes and is associated with fewer perioperative morbidity and shorter hospital stay compared with an open thoracotomy lobectomy (2).
Intubated general anesthesia with one-lung ventilation through a double-lumen endotracheal tube is the conventional norm for thoracoscopic procedures (3). However, complications after intubation with a double-lumen tube are not negligible (3). We have therefore endeavored to develop a less invasive thoracoscopic technique, namely nonintubated VATS, for the management of lung tumors (4-14).
With over 1,000 cases in 8 years, our experience has demonstrated that nonintubated VATS is a safe and versatile procedure, including various pulmonary resections, mediastinal tumor excision, and management of pleural diseases (4-14). We herein present a case of nonintubated VATS left upper lobectomy for a 56-year-old female patient with cT1bN0 non-small cell lung cancer (NSCLC) to share the surgical and anesthetic techniques for nonintubated VATS based on our experience.
Patient selection and workup
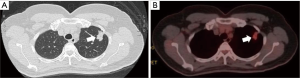
A 56-year-old female patient presented a left-sided lung nodule during a regular health examination. Her past medical history was not remarkable. Further imaging studies, including a thoracic computed tomography and a whole body positron emission tomography with technetium-99m, revealed a solitary peripheral speculated nodule in the left upper lobe with a maximal diameter of 27 mm and a hyper-metabolic character (Figure 1). They were highly suggestive of a cT1bN0 NSCLC.
Relevant data of a screening lung function test showed an FEV1 of 2.01 liters (104.6% predicted) and an FEV1/FVC of 82.0%. The patient was 152 cm tall with a body weight of 57 kg (body mass index: 24.7 kg/m2).
Both surgeon and anesthesiologist reviewed the patient’s medical records and thoracic images and agreed collaboratively to do a nonintubated approach with her consent before surgery.
Pre-operative preparation
Pre-operative preparation and intraoperative standard monitoring for nonintubated VATS are not different from intubated general anesthesia, including electrocardiography, blood pressure, body temperature and pulse oximetry. The patient was maintained with a target-controlled infusion of propofol, aiming for a bispectral index (BIS) value between 40 and 60. Without the use of muscle relaxants, the patient was kept in a spontaneous breathing status with supplemental oxygen via a ventilation facemask (Figure 2).
It is important to have a pre-planned protocol for airway management in case of needing conversion to tracheal intubation. For conversion, it is our practice to place a single-lumen endotracheal tube under the guidance of a bronchoscope, followed by insertion of a bronchial blocker for one-lung ventilation without changing the patient’s lateral decubitus position. The surgeon can cover the surgical wound with a transparent waterproof dressing. At the same time, a chest tube is left in the pleural space to facilitate re-expansion of the previously collapsed lung.
Procedure
Steps in our nonintubated procedures are briefly listed below:
- Intravenous sedation to deep general anesthesia with BIS-guided target-controlled infusion of propofol;
- Lateral decubitus position;
- Regional anesthesia using intercostal blocks and intrathoracic vagal block;
- Left upper lobectomy and lymphadenectomy;
- Hemostasis, re-expansion of the operative lung and wound closure with chest tube drainage.
Left upper lobectomy and lymphadenectomy
We performed VATS lobectomy using a 3-port method. A thoracoscopy port in the midaxillary line and a working port in the auscultatory triangle were initially created after local infiltration with 2% lidocaine. After introducing an open pneumothorax to collapse the operative lung, we infiltrate the intercostal space with 0.5% bupivacaine (1.5 mL for each intercostal space from the third to the eighth intercostal space) under direct thoracoscopic view. Then an intrathoracic vagal block was produced with 3 mL of 0.5% bupivacaine instilled adjacent to the vagus nerve at the level of the aortopulmonary window (Figure 3).

Operative steps for left upper lobectomy and mediastinal lymphadenectomy are listed below:
- Divide fissure between left upper lobe and left lower lobe using an endo-GIA;
- Divide lingular artery to left upper lobe and then lingular vein drained from left upper lobe using an endo-GIA;
- Divide proper vein drained from left upper lobe and then proper artery to the left upper lobe using an endo-GIA;
- Divide the bronchus to left upper lobe using an endo-GIA;
- Remove the divided left upper lobe into a protective bag;
At the end of the procedure, the operative lung was manually expanded via positive-pressure facemask ventilation to check for the presence of air leak. A single 28 F chest tube was placed through the lowest incision.
Role of team members
Like as any surgical procedure, nonintubated VATS requires a closely cooperative teamwork to facilitate efficiency and safety in the operating room.
The surgical team consists of:
- Surgeon and assistant;
- Anesthesiologist and nurse anesthetist;
- Scrub nurse and circulating nurse.
The surgeon should be the leader and direct other team members to ensure well communication and coordination of care in the operating room. The surgeon should be trained in all VATS techniques and should be able to resolve unexpected intraoperative complications, including an emergency thoracotomy.
The anesthesiologist is responsible for monitoring and maintaining the cardiopulmonary stability during a nonintubated procedure. Administration of intravenous anesthetics and opioid should be carefully titrated to avoid excessive suppression of respiratory function. Additionally, the anesthesiologist should pay close attention to the surgical environment and keep close communication regarding the respiratory pattern and other relevant safety issues with the surgeon. In a case of emergency conversion, the anesthesiologist should be able to secure the unprotected airway promptly and safely.
Postoperative management
To facilitate early recovery after VATS, postoperative management should focus on pain management, aggressive pulmonary physiotherapy, and early mobilization. She resumed oral intake 2 hours after surgery with oral nonsteroidal analgesics (Celebrex® and Ultracet®). There was neither air leak nor excessive pleural effusion; so that the chest tube was removed on postoperative day 2. She was discharged on postoperative day 3 after an uneventful postoperative course.
Tips, tricks, and pitfalls
- We suggest a cooperative and well-communicating thoracic surgical team, comprising of thoracic surgeon and anesthesiologist, is essential. Patients should be carefully selected in the early learning phase. It would be less stressful to start from simple peripheral wedge resection or management of pleural disease to get familiar with the surgical environment in a spontaneous breathing thoracic patient (14);
- We found that overweight patients (body mass index >26 kg/m2) are usually abdominal breather and are more likely to have vigorous diaphragmatic movement during nonintubated VATS. We would suggest that non-obese patients would be more suitable for a nonintubated VATS procedure (16);
- Transient tachypnea can occur at the beginning of iatrogenic open pneumothorax, which can get worse due to inadequate pain relief. Careful titration of propofol concentration or incremental use of opioid can suppress the ventilatory response and facilitate the collapse of the operative lung for a favorable surgical environment (16,17);
- An intrathoracic vagal block is effective to abolish the cough reflex, however, surgeons are still reminded to retract the lung and manipulate the hilum gently. In cases of dissection of sub-carinal lymph nodes, contralateral main bronchus can be occasionally irritated, which might induce transient coughing and jeopardize the safety (11);
- Oxygenation is mostly satisfactory after supplemental oxygen during spontaneous one-lung breathing but mild to moderate hypercapnia may occur because of carbon dioxide rebreathing. High flow nasal oxygen can be a useful device to improve oxygenation during nonintubated VATS. However, continuous extreme high flow rate (50–70 L/min) in the beginning of open pneumothorax can slow the collapsing rate of the operative lung;
- Although the incidence of conversion to intubated general anesthesia or thoracotomy is low, a conversion protocol in cases of failed nonintubated method should be prepared in advance and be performed without hesitation to decrease the risk of emergency intubation and complications.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Gonzalez-Rivas D, Aymerich H, Bonome C, et al. From Open Operations to Nonintubated Uniportal Video-Assisted Thoracoscopic Lobectomy: Minimizing the Trauma to the Patient. Ann Thorac Surg 2015;100:2003-5. [Crossref] [PubMed]
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- Knoll H, Ziegeler S, Schreiber JU, et al. Airway injuries after one-lung ventilation: a comparison between double-lumen tube and endobronchial blocker: a randomized, prospective, controlled trial. Anesthesiology 2006;105:471-7. [Crossref] [PubMed]
- Wu CY, Chen JS, Lin YS, et al. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann Thorac Surg 2013;95:405-11. [Crossref] [PubMed]
- Tsai TM, Chen JS. Nonintubated thoracoscopic surgery for pulmonary lesions in both lungs. J Thorac Cardiovasc Surg 2012;144:e95-7. [Crossref] [PubMed]
- Hung WT, Hsu HH, Hung MH, et al. Nonintubated uniportal thoracoscopic surgery for resection of lung lesions. J Thorac Dis 2016;8:S242-50. [PubMed]
- Hung MH, Hsu HH, Chen KC, et al. Nonintubated thoracoscopic anatomical segmentectomy for lung tumors. Ann Thorac Surg 2013;96:1209-15. [Crossref] [PubMed]
- Hung MH, Hsu HH, Chan KC, et al. Non-intubated thoracoscopic surgery using internal intercostal nerve block, vagal block and targeted sedation. Eur J Cardiothorac Surg 2014;46:620-5. [Crossref] [PubMed]
- Hung MH, Cheng YJ, Hsu HH, et al. Nonintubated uniportal thoracoscopic segmentectomy for lung cancer. J Thorac Cardiovasc Surg 2014;148:e234-5. [Crossref] [PubMed]
- Hung MH, Cheng YJ, Chan KC, et al. Nonintubated uniportal thoracoscopic surgery for peripheral lung nodules. Ann Thorac Surg 2014;98:1998-2003. [Crossref] [PubMed]
- Hung MH, Chan KC, Liu YJ, et al. Nonintubated thoracoscopic lobectomy for lung cancer using epidural anesthesia and intercostal blockade: a retrospective cohort study of 238 cases. Medicine (Baltimore) 2015;94:e727. [Crossref] [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic surgery using regional anesthesia and vagal block and targeted sedation. J Thorac Dis 2014;6:31-6. [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis 2012;4:347-51. [PubMed]
- Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg 2011;254:1038-43. [Crossref] [PubMed]
- Hung MH, Yang SM, Chen JS. Nonintubated thoracoscopic left upper lobectomy for lung cancer. Asvide 2017;4:029. Available online: http://www.asvide.com/articles/1335
- Yang JT, Hung MH, Chen JS, et al. Anesthetic consideration for nonintubated VATS. J Thorac Dis 2014;6:10-3. [PubMed]
- Wang ML, Hung MH, Chan KC, et al. Intraoperative multiple intercostal nerve blocks exert anesthetic-sparing effect: A retrospective study on the effect-site concentration of propofol infusion in nonintubated thoracoscopic surgery. Acta Anaesthesiol Taiwan 2016;54:77-80. [Crossref] [PubMed]
Cite this article as: Hung MH, Yang SM, Chen JS. Nonintubated video-assisted thoracic surgery lobectomy for lung cancer. J Vis Surg 2017;3:10.