Video-assisted thoracoscopic surgery and open chest surgery in infectious lung diseases
Introduction
Video-assisted thoracoscopic surgery (VATS) for infection or inflammatory conditions is uncommon compared to lung cancer. In most developed countries less than 5% of all VATS procedures are for infective/inflammatory conditions. However VATS is technically more challenging in this situations and are more prone to postoperative complications like
- Hemorrhage;
- Prolonged air leak;
- Pleural space problems for prolonged air leak and incomplete expansion of lung;
- Thoracic empyema due to pleural space problems and contamination of pleural space from spillage of infected material;
- Respiratory failure from spillage of infected material to contralateral lung or lobe;
- Bronchial dehiscence with bronchopleural fistula.
Therefore the principles of VATS for infectious diseases are the precise accurate surgical removal of the diseased infected lung and prevention of the above complications by minimally invasive technique (1,2).
Anaesthetic management
Anaesthetic management is very crucial for safe conduct of VATS for infective or inflammatory conditions (3). VATS surgery for infective conditions is fraught with dense chest wall and hilar adhesions due to repeated infections; good single lung isolation ensures good exposure to all parts of the chest for accurate lysis especially the apex and diaphragm which are the most inaccessible through a thoracotomy. More importantly it prevents spillage of and contamination of secretions from infected lung segments to normal lung caused by manipulation and positioning at time of surgery. This is ensured by suctioning of secretions in the airway and accurate single lung isolation under bronchoscopic guidance. In adults isolation can be obtained by double lumen endotracheal tube and in children small adults by bronchial blockers (Figure 1). A left-sided double lumen endotracheal tube is routinely used except during a left pneumonectomy when a right sided tube is preferred.
In bilateral bronchiectasis during surgery it is important to prevent accumulation of secretions in the non- operative diseased lung which can cause troublesome oxygen desaturation. This can be prevented by frequent bronchoscopic bronchial toilet.
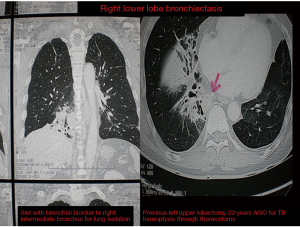
In bilateral synchronous lung resections usually in bronchiectasis a preoperative V/Q scan would help in choosing the side to be operated on first. Based on the V/Q scan the least diseased part or the better functioning lung should be operated on first to allow for safe single lung ventilation when operating on the more diseased side subsequently. In some staged cases where a previous contralateral lobectomy has been done single lung ventilation can be obtained by applying CPAP (5–10 mm) (Figure 1) to the operative lung or selective bronchial blockage of the intermediate bronchus to allow ventilation of the right upper lobe bronchus while surgery is conducted on either the lower or middle lobes (Figure 2).

Procedure and preference card
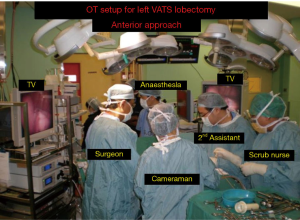
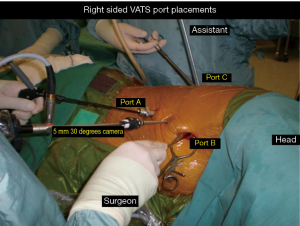
The VATS technique used is a totally endoscopic procedure performed by directly watching a TV monitor. The following non rib spreading ports are placed, a 5 mm 30 degrees camera placed over the major fissure at 5th intercostal space, a 5 mm retraction port placed at the 6th/7th intercostal space posterior and inferior to the scapula tip, a 10–15 mm utility working port at the 6th/7th intercostal space anterior axillary line and a 5 mm port at the 3rd intercostal space. The lobectomy is done through an anterior approach where the surgeon and camera assistant stands in front of the patient with the 2nd assistant and scrub nurse on the opposite side. The port placements and the position of the team remains the same for all lobes of both lungs and the camera stays in the same position throughout the procedure. All lobar vessels are individually dissected and divided (Figures 3,4).
Tips and pitfall
- Dense adhesions. Meticulous and complete lysis of all adhesions is mandatory after major lung resections for optimal exposure of the major hilar structures and completes reexpansion of the lung to prevent pleural space problems. Dense vascular adhesions from repeated previous infections precluding entry into chest via small ports. This can be overcome by making a small 10–15 mm initial port incision and lysis the adhesions under direct camera vision. Lysis of adhesions is performed with blunt and sharp dissection depending on the vascularity and density of the adhesions. If the adhesions are flimsy and nonvascular blunt dissection with a “peanut” dissector is optimal. If the adhesions are vascular and dense a 5 mm hook dissector or energy source would be optimal to minimise air leak and bleeding. Hemostasis should be absolute to minimise blood loss and for optimal visualisation. In some cases when the lung is completely adherent or invasion into the chestwall especially near vital structures extrapleural dissection should be done to obtain a safe plane of dissection. Troublesome bleeding from chestwall can be controlled with argon beam coagulator or more simply by using hydrogen peroxide over the areas of diffuse oozing (Figures 5-7);
- Fused fissures. Due to repeated infections fused and thick scarred fissures can be a problem when performing lobectomies. The safest technique is to do a fissureless lobectomy where the fissure is left last for division after all the main hilar structures have been divided and also resection of the non-involved ide of the fissure. Figures 2,4, demonstrates a right VATS upper lobectomy in 44 years old lady with post tuberculous aspergilloma with repeated history of hemoptysis. It demonstrates lysis of dense vascular adhesions through VATS. Figures 5,8 highlights a fissureless lobectomy as the fissures were completely fused and very thick. The fissures were divided last with a green reload endostapler rather than blue due to the thick fused fissures;
- Hypertrophied bronchial vessels can cause troublesome bleeding during VATS and postperatively if not adequately managed. All large bronchial vessels should be clipped and not cauterized as they can be a cause for bleeding post operatively;
- Hypertrophied and calcified lymph nodes can be a big problem in VATS lobectomies for inflammatory conditions especially in the dissection of the pulmonary artery branches. Dissection of enlarged reactive lymph nodes is easy to manage if the dissection of the pulmonary artery is kept within the vascular sheath of the artery as the lymph nodes always outside on this plane. However in calcified nodes this plane is lost and it is best to obtain and proximal and distal control of the artery before dissection or division of this vessels;
- In cases where the hilum has been scarred from repeated infections and previous surgery access to the pulmonary structures can be difficult and hazardous especially when performing completion pneumonectomy. This can be overcome by opening the pericardium to obtain control of these structures. Figure 7 a 64-year-old female non-smokers underwent a right middle and lower lobectomy by thoracotomy in May 2014 in another institution for T2N0M0 adenocarcinoma of the right lower lobe. Post-operative stay was prolonged for 10 days due to possible non re-expansion of lung with prolong chest tube placement. Repeat PET CT scan thorax in Oct 2014 showed a local recurrence in the upper lobe at the previous staple line with loculated hydro-pneumothorax. Patient was referred to our institution for second opinion. As lung function was adequate and there were no other evidence of metastatic disease patient underwent a right VATS completion pneumonectomy. The surgery proved challenging due to dense chest wall/diaphragm adhesions and a frozen hilum due to relatively recent history of surgery and empyema. The pericardium was identified and opened. The aorta was identified and the superior pulmonary vein was identified and divided intrapericardially. Unfortunately due possible previous pleural infection the pericardium over the SVC, right main pulmonary artery was completely obliterated and fibrotic. Decision was made to divide the azygos vein and then mobilise and divide the right main stem and intermediate bronchus stump first. The posterior wall of the pulmonary artery behind the bronchial stump was then identified and traced anteriorly to the pericardium. The pericardium was then opened and the right main pa mobilises, taped and divided with endostaplers. The remnant lung and bronchial stump was then mobilised off the subcarinal space and esophagus carefully. No attempt was made to dissect the lung off the inferior vein extrapericardially as there was a risk of injury. The lower lobe pulmonary vein was restapled intrapericardially to avoid this injury. The lung was removed via enlarging the 10 mm port. Hemostasis was secured. The right bronchial stump margin was send for frozen section analysis and once confirmed to be clear of cancer, was closed with Polydiaxone (PDS) 40 interrupted sutures. Air leak check of the stump was done under water pressure of 30 cm of water pressure, Tissue glue was applied over the bronchial stump. The pericardial defect was closed with bovine pericardium. One chest tube was placed which was removed 48 hours later. Blood lost was 700 mL. Operative time was 380 minutes. Hospital stay was 5th pod (Figure 9);
- Bronchial stump dehiscence. Bronchial stump dehiscence can be a problem when major lung resections are done for infective or inflammatory conditions. This can be prevented by minimising bronchial devascularisation and buttressing of bronchial stump with surrounding tissues like pleural flap, thymus, intercostal muscle or pericardium (Figure 5). Post-operative empyema especially in pneumonectomy can be prevented by avoiding contamination of the pleural space by early stapling of the bronchus, prevention of spillage of infected lung tissue by careful dissection and mobilisation of the lung and removal of the specimen by an endobag;
- Persistent pleural space. A persistent pleural space can be a problem after surgery for chronic infectious lung conditions. This inflammatory fibrosis of the lung parenchyma and chest-wall can prevent full re-expansion of underlying lung with can result in persistent air leaks and empyema. Lysis of adhesions and complete mobilisation of the whole lung is done to allow full re-expansion of the remainder lobes to fill the residual pleural space. In upper lobectomy division of inferior pulmonary ligament and in others pleural tenting or muscle flap may be necessary.




Patient selection
Indications for VATS surgery for infectious diseases is to treat failed or no medical therapy, development of acute or chronic complications and inability to rule out malignancy in a mass or cavitary lesion. Common types of infectious diseases can be either pyogenic, chronic granulomatous or secondary infection of preexisting congenital or developmental lung conditions (9).
Preoperative preparation and timing of surgery
- Surgery should be done whenever possible electively or semi-electively after control of acute sepsis. Active and acute infections should be treated aggressively before surgery with antibiotics based on cultures, drainage of abscess and chest physiotherapy. Surgery for bronchiectasis should be done only between exacerbations. If malnourished supplemental nutrition is necessary before surgery (9);
- In the case of massive hemoptysis from bronchial artery bleeding emergent embolization with stabilisation of patient and prevention of spillage of blood from diseased to contralateral lung should be done before performing surgery safely on a semi elective basis (10).
Procedures
- Acute complication of pyogenic infection = thoracic empyema. Thoracic empyema is divided into three stages, exudative, fibrinopurulent and organised chronic stages. VATS decortication generally indicated for fibrinous and chronic stages. Principles of VATS decortication includes drainage of all loculated pus and fibrin, removal of all thickened visceral and parietal pleura entrapping the lobes or lung with full re-expansion of the whole lung and no residual pleural space (11) (Figure 10);
- Chronic complication of pyogenic infection =limited or localised Bronchiectasis. Indications for surgery in bronchiectasis is confined to patients with unilateral or bilateral limited localised disease with recurrent hemoptysis, repeated infections or chronic lung abscess who have failed medical therapy (Figures 2,11). Figure 2 shows a 45-year-old gentleman who 20 years ago had a left lower lobectomy for bronchiectasis through a thoracotomy. Now presents with right lower lobe bronchiectasis. To obtain single lung ventilation the right intermediate bronchus was isolated with a bronchial blocker. This allowed the right upper lobe to be ventilated and ensuring adequate saturation and working space for safe conduct of the right VATS lower lobectomy (12-14);
- Sequelae of chronic granulomatous lung infections (2,15-20):
- Chronic tuberculous empyema with entrapped lung despite adequate treatment;
- Aspergilloma in tuberculosis (TB) cavity. Surgery is indicated for chronic symptoms, complex aspergilloma and recurrent or massive hemoptysis (Figure 2);
- TB bronchial stricture (Figure 12);
- Destroyed lung parenchyma (Figure 12);
- Localised resistant or recurrent chronic infections (MDRT TB, atypical mycobacterial infections, fungal infections).
- Infective complications of congenital conditions:

Post-operative management
Post-operative care includes adequate pain control, pulmonary toilet, chest physiotherapy, early mobilisation and maintenance of nutrition. Fluid restriction and prevention of arrhythmias are additional measures in patients undergoing pneumonectomy. Culture results obtained from resected tissue are used to adjust antimicrobial therapy if indicated (2).
Discussion
Although open thoracotomy continues to be the preferred choice for inflammatory conditions, with increasing experience of VATS for lung cancer it is increasingly used for inflammatory conditions. Its advantages over thoracotomy are the optics, magnification and lighting which give excellent exposure to all parts of the chest cavity. Shorter hospital stay and fewer complications have been reported as the surgery is better tolerated due to less chest wall injury and better preservation of immediate postoperative lung function. It should be attempted by only experienced VATS surgeons after overcoming the learning curve in lung cancer surgery in carefully selected case. At present time VATS is suitable for inflammatory conditions with minimal scarring, absent of dense adhesions in the fissure or perihilar region with absence of calcified/enlarged lymph nodes in the vicinity of major pulmonary vessels. In one report conversion to open occurred in 5% of patients with negligible mortality and 8% morbidity due to prolonged air leak, atelectasis and effusions (1).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mitchell JD, Yu JA, Bishop A, et al. Thoracoscopic lobectomy and segmentectomy for infectious lung disease. Ann Thorac Surg 2012;93:1033-9; discussion 1039-40. [Crossref] [PubMed]
- Reed CE. Pneumonectomy for chronic infection: fraught with danger? Ann Thorac Surg 1995;59:408-11. [Crossref] [PubMed]
- Benumof JL, Alfery DD. Anaesthesia for thoracic surgery. In: Miller RD. Anaesthesia. 5th edition. Philadelphia: Churchill Livingstone, 2000:1665-75.
- Thirugnanam A. Right video-assisted thoracoscopic surgery (VATS) lower lobectomy for bronchiectasis. Asvide 2016;3:514. Available online: http://www.asvide.com/articles/1289
- Thirugnanam A. Right upper lobe fissureless lobectomy for aspergilloma with bronchial buttressing. Asvide 2016;3:515. Available online: http://www.asvide.com/articles/1290
- Thirugnanam A. Left video-assisted thoracoscopic surgery (VATS) lower lobectomy for infected congenital cystic adenomatoid malformation (CCAM). Asvide 2016;3:516. Available online: http://www.asvide.com/articles/1291
- Thirugnanam A. Left video-assisted thoracoscopic surgery (VATS) upper lobectomy for infected congenital cystic adenomatoid malformation (CCAM) in a child. Asvide 2016;3:517. Available online: http://www.asvide.com/articles/1292
- Thirugnanam A. Completion pneumonectomy. Asvide 2016;3:518. Available online: http://www.asvide.com/articles/1293
- Merritt RE, Shrager JB. Indications for surgery in patients with localized pulmonary infection. Thorac Surg Clin 2012;22:325-32. [Crossref] [PubMed]
- Lim Yp, Wong D, Agathian T. Management of life-threatening hemoptysis. Asian Cardiovas Thorac Ann 2001;9:200-3. [Crossref]
- Chan DT, Sihoe AD, Chan S, et al. Surgical treatment for empyema thoracis: is video-assisted thoracic surgery "better" than thoracotomy? Ann Thorac Surg 2007;84:225-31. [Crossref] [PubMed]
- Agasthian T, Deschamps C, Trastek VF, et al. Surgical management of bronchiectasis. Ann Thorac Surg 1996;62:976-8; discussion 979-80. [Crossref] [PubMed]
- Agasthian T. Results of surgery for bronchiectasis and pulmonary abscesses. Thorac Surg Clin 2012;22:333-44. [Crossref] [PubMed]
- Zhang P, Zhang F, Jiang S, et al. Video-assisted thoracic surgery for bronchiectasis. Ann Thorac Surg 2011;91:239-43. [Crossref] [PubMed]
- Dewan RK. Surgery for pulmonary tuberculosis - a 15-year experience. Eur J Cardiothorac Surg 2010;37:473-7. [PubMed]
- Kim YT, Kim HK, Sung SW, et al. Long-term outcomes and risk factor analysis after pneumonectomy for active and sequela forms of pulmonary tuberculosis. Eur J Cardiothorac Surg 2003;23:833-9. [Crossref] [PubMed]
- Ichinose J, Kohno T, Fujimori S. Video-assisted thoracic surgery for pulmonary aspergilloma. Interact Cardiovasc Thorac Surg 2010;10:927-30. [Crossref] [PubMed]
- Jungraithmayr W, Hasse J, Olschewski M, et al. Indications and results of completion pneumonectomy. Eur J Cardiothorac Surg 2004;26:189-96. [Crossref] [PubMed]
- Park CK, Jheon S. Results of surgical treatment for pulmonary aspergilloma. Eur J Cardiothorac Surg 2002;21:918-23. [Crossref] [PubMed]
- Mitchell JD, Bishop A, Cafaro A, et al. Anatomic lung resection for nontuberculous mycobacterial disease. Ann Thorac Surg 2008;85:1887-92; discussion 1892-3.
- Thirugnanam A. Video-assisted thoracoscopic surgery (VATS) pneumonectomy for destroyed lung secondary to tuberculosis (TB) stricture. Asvide 2016;3:519. Available online: http://www.asvide.com/articles/1294
Cite this article as: Thirugnanam A. Video-assisted thoracoscopic surgery and open chest surgery in infectious lung diseases. J Vis Surg 2017;3:3.