Simultaneous laparoscopic resection of distal pancreas and liver nodule for pancreatic neuroendocrine tumor
Introduction
Laparoscopic distal pancreatectomy (LDP) was first described by Cuschieri et al. (1) for benign diseases in 1996; in the same year, Gagner et al. (2) reported their early experience with eight LDP performed in patients with islet cell tumors. Nowadays, LDP is the procedure of choice for small lesions of the pancreatic body-tail of various nature.
In the literature there are many papers that demonstrate the advantages of LDP versus open distal pancreatectomy (ODP) in terms of severe complication reduction according to Clavien-Dindo classification (3), reduction of blood loss and shorter length of hospital stay (4-8). There are no differences between the two techniques in terms of postoperative pancreatic fistula (POPF) development.
Published data on oncologic radicality are limited as the mininvasive technique is mainly reserved to benign or borderline disorders, leading to relevant biases on the results.
Randomized controlled trials are needed to validate the effective advantages of LDP over ODP (9-11).
Patient selection and work-up
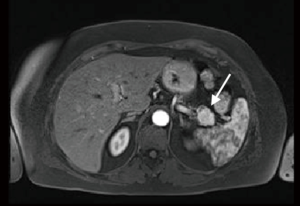
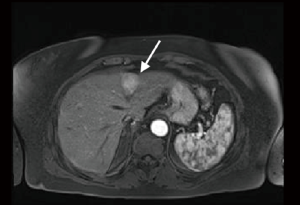
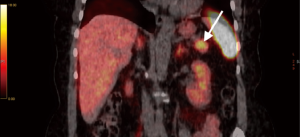
A 65-year-old woman, with no previous medical history, underwent a CT scan for living organ donation evaluation that showed a 3 cm hypervascular lesion in the pancreatic tail and a 3 cm slightly hypervascular nodule in segment 2 of the liver. Magnetic resonance imaging (MRI) with extracellular contrast confirmed the presence of both pancreatic and hepatic lesions (Figures 1,2). The pancreatic lesion was hypointense on both T1w and T2w phases, slightly hyperintense on T2w phase, high signal in DWI sequence, hyperintense on postcontrast arterial phase and isointense on venous phase. The hepatic lesion appeared isointense on precontrast T1w phase, very slightly hyperintense on T2w fs phase, hyperintense on postcontrast T1 phase and it showed slightly restricted diffusion. Scans were suggestive of neuroendocrine tumor of the pancreas with undetermined liver nodule. 68Ga-DOTATOC-PET revealed high uptake of the radiotracer in a 30 mm area of the pancreatic tail (Figure 3). Laparoscopic exploration and resection of both pancreatic and liver lesions was planned.
Pre-operative preparation
The patient fasted for 12 hours before surgery. The operation was performed under general anesthesia with endotracheal intubation. A 16 F gastric decompression tube and urinary catheter were placed. Prophylactic third generation cephalosporine was administered intravenously on induction.
Equipment preference card
High definition laparoscopic video system, pneumoperitoneum system, ultrasonic dissector, laparoscopic instruments including atraumatic graspers, scissors, clipping devices, surgical stapler and plastic specimen bag were prepared.
Procedure (Figure 4)

The patient was placed in a supine position with abducted spreaded legs. The chief surgeon stood between the legs. The first assistant and camera operator stood on the right side of the patient, the second assistant stood on the left side of the patient as well as the laparoscopy screen. A 10-mm trocar was placed in periumbilical region and pneumoperitoneum was created with open technique. The intra-abdominal pressure was maintained at 12 mmHg. The other trocars were placed at right-upper and left-upper quadrants (15 mm) and in epigastric region (5 mm).
Laparoscopic exploration was performed and the gastrocolic ligament was opened with ultrasonic dissector. An intraoperative ultrasound confirmed the body-tail lesion and the hepatic 3 cm nodule in segment 2 of uncertain nature. The peritoneum under the inferior margin of the pancreas was dissected and pancreas was mobilized, splenic vein was discovered and section was performed after clips positioning (Weck® Hem-o-lok® Teleflex Incorporated, Morrisville, NC, USA). The splenic artery was identified at the superior edge of the pancreas and sectioned after clips positioning. Furthermore the pancreas was sectioned with a laparoscopic stapler (EndoGIA Covidien Inc., Mansfield, MA, USA) and mobilized from the body to the tail. Splenic isolation completed the distal pancreatectomy. The surgical specimen was immediately put into a plastic specimen bag and retrieved through a small Pfannenstiel incision. Haemostasis of the surgical field was secured.
The hepatic lesion in S2 was entirely resected with safe margins with ultrasonic dissector with a satisfying haemostasis. Prophylactic cholecystectomy was performed.
Three drainages were placed: one in the subhepatic region, one in the splenic region and one close to the pancreatic stump.
Role of team member
- Dr. Nicola Passuello: Trainee;
- Dr. Michele Valmasoni: Surgeon;
- Dr. Gioia Pozza: Trainee;
- Dr. Elisa Sefora Pierobon: Surgeon;
- Dr. Alberto Ponzoni: Radiologist;
- Dr. Cosimo Sperti: Surgeon.
Post-operative management
Short course 3rd generation cephalosporin was administered. Gastric tube was removed on POD 2 and the patient started eating on the same day. Drain amylase levels were checked on POD 1, 3 and 5 and were always negative. Both the subhepatic and splenic drains were removed on POD 4 while the remaining was removed on POD 6. The patient was discharged on the same day.
Histopathological examination of the pancreatic specimen showed a neuroendocrine G2 tumor with no lymph node metastasis. Immunohistochemistry examination showed MIB1 3%, chromogranin A, beta-catenin and synaptophysin positivity. The hepatic nodule analysis demonstrated focal nodular hyperplasia.
Tips, tricks and pitfalls
Gastrocolic ligament opening must to be large in order to have space to insert the ultrasonic probe. Attention must be paid to right and left gastroepiploic arteries preservation, section of the short gastric vessels need to be carried out in order to mobilize the spleen from the stomach.
Ultrasound examination must be accurate in order to define the characteristics of the tumor, the relationship of the lesion with the Wirsung duct and, above all, with the splenic and mesenteric vessels.
The most challenging part of the procedure is the splenic vessels isolation: splenic vein must be isolated with caution at the inferior edge of the pancreas, and a sufficiently long portion of vein need to be dissected in order to clip the vessel in two points reducing the risks of bleeding. Furthermore, it is important to identify and section all the small single venous branches that come off the pancreas into the splenic vein.
An appropriate drainage positioning is eventually needed: in fact, a correct drainage of the pancreatic stump is a useful way of access for a radiological treatment in case of POPF development.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cuschieri A, Jakimowicz JJ, van Spreeuwel J. Laparoscopic distal 70% pancreatectomy and splenectomy for chronic pancreatitis. Ann Surg 1996;223:280-5. [Crossref] [PubMed]
- Gagner M, Pomp A, Herrera MF. Early experience with laparoscopic resections of islet cell tumors. Surgery 1996;120:1051-4. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Hasselgren K, Halldestam I, Fraser MP, et al. Does the Introduction of Laparoscopic Distal Pancreatectomy Jeopardize Patient Safety and Well-Being? Scand J Surg 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Nappo G, Perinel J, El Bechwaty M, et al. Minimally Invasive Pancreatic Resection: Is It Really the Future? Dig Surg 2016;33:284-9. [Crossref] [PubMed]
- Mehrabi A, Hafezi M, Arvin J, et al. A systematic review and meta-analysis of laparoscopic versus open distal pancreatectomy for benign and malignant lesions of the pancreas: it's time to randomize. Surgery 2015;157:45-55. [Crossref] [PubMed]
- Sánchez-Cabús S, Adam JP, Pittau G, et al. Laparoscopic left pancreatectomy: early results after 115 consecutive patients. Surg Endosc 2016;30:4480-8. [Crossref] [PubMed]
- Stauffer JA, Coppola A, Mody K, et al. Laparoscopic Versus Open Distal Pancreatectomy for Pancreatic Adenocarcinoma. World J Surg 2016;40:1477-84. [Crossref] [PubMed]
- de Rooij T, Jilesen AP, Boerma D, et al. A nationwide comparison of laparoscopic and open distal pancreatectomy for benign and malignant disease. J Am Coll Surg 2015;220:263-270.e1. [Crossref] [PubMed]
- Sulpice L, Farges O, Goutte N, et al. Laparoscopic Distal Pancreatectomy for Pancreatic Ductal Adenocarcinoma: Time for a Randomized Controlled Trial? Results of an All-inclusive National Observational Study. Ann Surg 2015;262:868-73; discussion 873-4. [Crossref] [PubMed]
- Riviere D, Gurusamy KS, Kooby DA, et al. Laparoscopic versus open distal pancreatectomy for pancreatic cancer. Cochrane Database Syst Rev 2016;4:CD011391. [PubMed]
- Passuello N, Valmasoni M, Sperti C, et al. Simultaneous laparoscopic resection of distal pancreas and liver nodule for pancreatic neuroendocrine tumor. Asvide 2016;3:487. Available online: http://www.asvide.com/articles/1262
Cite this article as: Passuello N, Valmasoni M, Pozza G, Pierobon ES, Ponzoni A, Sperti C. Simultaneous laparoscopic resection of distal pancreas and liver nodule for pancreatic neuroendocrine tumor. J Vis Surg 2016;2:176.


