Video-assisted thoracic surgery and open chest surgery in lung cancer treatment: present and future
“The heart alone of all viscera will not withstand surgery; no new method and no new techniques will overcome the natural obstacles surrounding a wound of the heart”—Sir Stephen Paget, 1896
In the same year Rehn of Frankfort proved him wrong by successfully suturing a stab wound.
Introduction
There is no question that minimally invasive techniques have had a profound impact on surgery in general, With respect to thoracic surgery, it is now over 20 years since video-assisted thoracic surgery (VATS) techniques were first reported for lobectomy (1-3). Since then a plethora of papers have examined many aspects of this approach. Nevertheless, lobectomy via open thoracotomy continues to be in widespread use as well.
This paper does not seek to recount the history of the development of VATS surgery. Instead it is an assessment of the current state with regards to open and VATS approaches for lung cancer resection. The aim is to shed light on the reasons for different rates of uptake of VATS in different regions. This sets the stage for a discussion of considerations regarding what should be the appropriate place for VATS vs. open surgery. Finally, we offer some speculation on changes that may occur in the future.
General background
Changes in the landscape of thoracic diseases
Thoracic surgery has changed over time. At one time a very large thoracotomy incision with rib removal was standard; this is now only rarely used in exceptional circumstances. A variety of smaller muscle sparing incisions have become the norm for open surgery, and a number of VATS approaches have also been widely adopted. Training in thoracic surgery has become more organized, and there is a greater prevalence of people who have been specifically trained in thoracic surgery. There are different options for pain control. Management of chest drainage has changed. The operative mortality has declined, and the hospital stay has also decreased significantly.
Many additional changes (beyond technical surgical advances) have occurred that have an impact on thoracic surgery. Anesthetic management has changed; patients are able to be extubated easily with less need for intensive care. The culture has changed from an emphasis on bedrest to an emphasis on early ambulation. In general the expectations of patients and their families has changed, although this varies significantly by country and based on the structure of the healthcare system. Management of non-pulmonary conditions has also improved (e.g., cardiovascular disease) – with the effect that patients (e.g., older age) are now considered for thoracic resection that in the past would have been excluded.
There have also been changes in the nature and spectrum of disease encountered in thoracic surgery. Fewer patients are seen now than in past decades with hilar scarring from earlier infections, although there is significant variation in these rates according by region. This is due to a decreased prevalence of such infections, as well as the fact that the lifespan of many patients with such past exposures has been reached exceeded. This affects the technical difficulty of performing lung resection. Lung cancer has shifted to more peripheral tumors, and a greater incidence of tumors that are smaller at the time of detection, due to an increased prevalence of CT imaging in general as well as for lung cancer screening.
Factors inherent in the development and adoption of new technology
The only thing that is constant is change. Furthermore, as Charles Darwin wrote “It is not the strongest of the species that survives, nor the most intelligent; it is the one that is most adaptable to change”. The pace of change and the evolution of technology is increasing at an ever faster rate. The need to change is clear, but we must grapple with identifying which changes are destined to become dominant and hence should be embraced and which are temporarily enticing but fleeting.
New innovations are often described in terms taken form the business world as “sustaining” or “disruptive” innovations. Sustaining innovations are ones that refine an existing technology; these can be either evolutionary (a logical progression) or revolutionary (unexpected). A disruptive innovation however is one that creates a new market by providing a different set of values, which ultimately overtakes an existing market. Disruptive technologies are usually not radically technologically different; instead it is the business model that the technology enables that creates the disruptive impact. The automobile is not considered disruptive; it was initially only an expensive alternative to the horse drawn carriage. However, the mass production of cheap Model T Ford cars on an assembly line not only replaced the carriage, but brought availability of self-powered transportation to a vast number of people and spawned the development of the fossil fuel industry, roads and many other developments.
Adoption of new technology is often categorized as follows: new innovations are first adopted by a small group of “innovators”, then “early adopters” who are part of defining the value. After this comes the “early majority”—people who wait for data that proves the value of an innovation. The “late majority” waits until it has become an established standard; finally the “skeptics” continue to resist new technologies until very late, often for personal reasons. This reflects human nature; some people are excited by new things, but for most of us learning new skills require overcoming some inertia.
Drivers of adoption of new technologies are data demonstrating its value, evolutionary advancements that make it easier to use, and the availability of training allowing dissemination of the skill needed. Other factors are also important drivers of the adoption of medical technologies. It has often been demonstrated that patients will chose a less invasive approach, even if it is less effective (up to a point). The experience of coronary stenting vs bypass surgery is a good example. Another important concept is that “user-friendliness” trumps effectiveness; if a new technique is hard to perform or learn, adoption will be limited. Furthermore, if the skills are difficult to learn it is likely they will never become widely disseminated and available. Finally cost is an important component. However, this is heavily influenced by who is directly bearing the cost, which varies according to the healthcare system. Patients are not influenced by the cost unless they are directly responsible; they may drive adoption of a new technology regardless of the costs to the health care system. Thus, while effectiveness is important, aspects such as lack of user-friendliness (for the patient or the physician), lack of availability, and cost can be the aspects that determine the fate of an innovation.
Adoption of a new technology and data defining its value do not always match, illustrating the adage that “perception is reality”. When awareness and familiarity of an innovation is limited, it is easy to cite reasons for not adopting it, regardless of the data. Once a “tipping point” has been reached, it becomes difficult to argue against it without knowing the relevant data. Often, however, the strength of the perception in favor of a new technology far outsteps the actual data.
Status of open and VATS resection for lung cancer
Proportion of cases by technique
The proportion of lobectomies performed open or by VATS varies over time and by region. Solid data is only available from a few countries (Figure 1). It is clear that the proportion of cases has been increasing over time. It is also clear that there are differences among countries and even by type of institution. The use of VATS is the highest in US institutions that contribute to the Society of Thoracic Surgeons (STS) database—these are primarily academic institutions with dedicated thoracic surgery programs. The US National Inpatient Sample (NIS) database shows a lower use of VATS; this database is designed to be a representative sample of US hospitals in general. In this database lobectomies that are performed by general and cardiac surgeons. (The NIS VATS data includes cases performed robotically, which have increased from 1.3% in 2009 to 9.7% in 2013.). Although national data is not available, it is estimated to be quite low in South America overall, but almost 50% in a few major medical centers (personal communication, Ricardo Terra, September 2016).
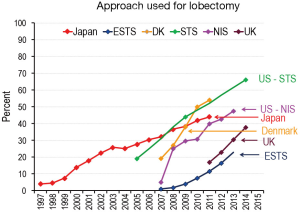
The penetration of VATS for lobectomy has been much more limited in Europe overall. However this varies significantly by country. In 2014, for example, VATS was used in 3.6% of lobectomies in Romania, 17% in Italy and France, and 27% in Belgium [data from the European Society of Thoracic Surgeons (ESTS) database]. Denmark has seen rapid adoption; this has been less so in the United Kingdom.
Data from Japan demonstrates a gradual slow increase in the use of VATS for lobectomy, beginning many years ago. This data includes the use of “hybrid” VATS (~30% of VATS cases), in which a camera is used partially as a light source and the resection is performed through a small thoracotomy incision, generally without rib spreading (4). National statistics are not available from China, but it is estimated that close to half of lobectomies in China in 2016 are performed by VATS, reflecting a very rapid adoption of this approach within the past 5 years (personal communication Alan Sihoe and Vincent Fang, September 2016).
Comparison of results of open vs. VATS lobectomy
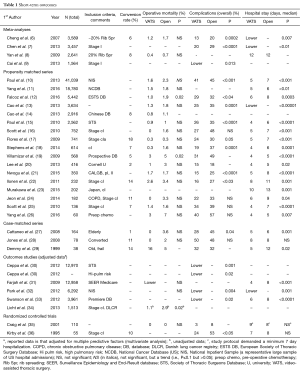
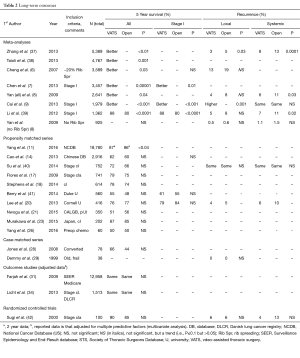
Since the first reports of VATS lobectomies in 1992 and 1993 (1-3), a great deal of experience has been accumulated. The data includes large database analyses with unmatched comparisons, meta-analyses, propensity matched studies, outcome studies with results adjusted for other factors and a few small randomized studies. This data is discussed in detail in a recent book chapterJeny (5); the major results are summarized briefly here, updated with some additional recent studies (Tables 1,2). Because patients are clearly selected for VATS vs. open lobectomy, the most reliable data comes from either matched comparisons or randomized studies.
Full table
Full table
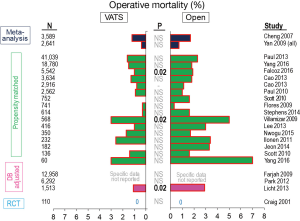
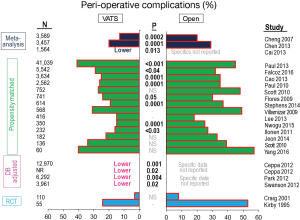
Operative mortality appears to be slightly less in VATS compared with open resections (Table 1, Figure 2). However, the difference is slight and not statistically significant in most matched comparisons, possibly reflecting the fact that statistical significance is hard to demonstrate when the incidence of an event (perioperative death) is very low. The rate of complications also is generally lower in VATS resections, and this is statistically significant in about half of the matched comparisons (Table 1, Figure 3). (It is hard to compare one study to another study because of differences in how complications are defined, but these definitions were consistent within each study.) The observation of fewer complications with VATS among matched comparisons or randomized trials is also seen when analyzing specific complications (e.g., arrhythmia, pneumonia, prolonged air leak, mechanical ventilation (5).
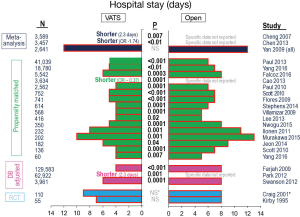
Hospital length of stay varies substantially between studies, reflecting many differences in societal norms and the structure of the health care system. Nevertheless, within almost every unmatched or matched comparison a statistically significant shorter length of stay is demonstrated for the VATS approach (Table 1, Figure 4). Postoperative pain is diminished during the first few weeks after VATS, but this difference dissipates thereafter (5,6). Limited data suggests better quality of life and more rapid return to independent functioning after VATS (5,6).
Data from a large number of unmatched, nonrandomized comparisons have shown no difference in mediastinal node staging between VATS and open lobectomy (14 studies, P=0.63) (6). This issue has also been addressed in two randomized and several prospective trials, which found no difference (9,38,43,44). A propensity matched study of the US National Cancer Database (NCDB) found a small but significant increase in the number of nodes examined during VATS vs. open resections (10.3 vs. 9.7, P<0.01) (45), but another metaanalysis specifically addressing node staging found no difference (37). Two recent propensity matched analyses of cI patients in the NCDB reached conflicting conclusions regarding nodal upstaging (11,45), suggesting that perceived differences may be a reflection of other factors and not actually the approach used. Taken together, the data suggest little inherent difference in the ability to carry out intraoperative staging between VATS vs open resections. While there is some data suggesting possible decreased N1 node assessments with VATS, there are others showing the opposite, and the validity and impact of any difference are unclear.
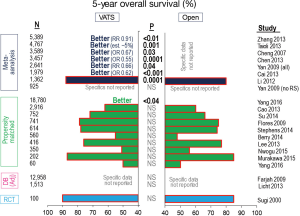
The ultimate goal of resection, of course, is long-term cure of cancer. Many unmatched comparisons of VATS vs. open resections suggest better long-term survival after VATS (Table 2). However, when propensity matching is used to correct for selection bias there is no difference in long-term outcomes (Table 2, Figure 5). This is also true when specifically examining local and systemic recurrence rates (5).
Discussion
Over 20 years since the advent of VATS lobectomy, the degree of penetration of VATS is highly variable. It has become quite common in the US, Japan and China, lower in Europe and varying by country. There are likely many factors at play, but the penetration seems to parallel how developed the economy is. This association is probably overly simplistic; factors such as how the health care system is organized and the types of cancers that are encountered are also likely to play a role. Finally it also reflects the local degree of experience and comfort with VATS, as demonstrated by the varying rates in different institutions in the US and UK.
What should the degree of penetration be? This is a difficult question to answer, since there are many factors at play. A justifiable reason for varying use is a difference in the types of tumors encountered (i.e., central, more advanced) and the incidence of hilar and mediastinal scarring. Differences in the type and stage of the tumors may be influenced by access to medical care and the general incidence of CT scanning. Health economic considerations can vary greatly. Whether VATS is more or less expensive that open lobectomy depends on the relative costs of staplers and equipment (video towers), OR time and days in the hospital. Furthermore, how the costs actually impact physicians, hospitals and patients depends on the structure of reimbursement. Cultural and regional differences are likely to play a large role as well. The distance needed to travel to home, societal expectations, and people’s willingness to deal with pain and limitations varies. For example, the use of narcotics in prospective studies after a standardized (measuring the incision, degree of rib spreading) open thoracotomy is ~35% at one month in the US, and ~10% at one week in China (46,47). Finally, the availability of instruments and equipment as well as skills can be a limiting factor. However, if medical, patient acceptance and economic factors favor VATS, then a difference in availability of skills and equipment will likely be overcome within a short time period.
At this point, one cannot argue that there is insufficient data to evaluate the value of VATS vs. open lobectomy. The available data demonstrates long term outcomes that are equal, and short term outcomes that are similar (perioperative mortality) or better (lower incidence of complications and length of stay). The degree of postoperative pain is diminished during the recovery period. The extent and consistency of data when controlling for potential confounding factors suggest that VATS is a viable alternative to open resection for lung cancer. The American College of Chest Physicians Evidence-Based Guideline for Lung Cancer suggests that VATS is preferred over an open approach to lobectomy/segmentectomy for clinical stage I lung cancer—in experienced centers (48). Finally, only strong data demonstrating inferiority could counter the fact that a minimally invasive approach has great appeal to patients.
There is no right proportion of lobectomies that should be performed by VATS. Each region will have to determine this individually. However, one should be careful to evaluate this objectively and base it on appropriate reasons. Factors such as lack of comfort with VATS lobectomy or equipment by themselves should probably spark an attempt to overcome them rather than justify accepting the status quo if other factors suggest that the penetration should be higher. If VATS is indeed appropriate, it is likely that patients will drive greater penetration of VATS. When the degree of penetration is variable and reflects primarily the availability of expertise, patients will tend to migrate to places where it is available. On the other hand, when the skills are generally available, a general consensus definition of the appropriate penetration of VATS in a region can emerge; when this has been achieved patients will largely accept the surgeon’s choice of approach according to this consensus.
Whether learning to perform VATS lobectomy is appropriate differs by region and setting. An important consideration is the learning curve; several investigators have found that the learning curve to achieve a reasonable comfort level with VATS lobectomy appears to be around 50 cases (14,16,19). One has to evaluate whether one’s situation will allow getting over this learning curve; if not, it may be more appropriate to focus on performing good quality open resection. The learning process can be approached as a gradual evolution, although there is a clear demarcation between VATS for wedge and pleural disease and for lobectomy. Transitioning to smaller incisions, performing some dissection via VATS, converting to an open approach when one’s comfort level is exceeded, and of course having a mentor contribute to making the learning process easier.
What type of new innovation is VATS lobectomy? It may not be appropriate to use terms derived from the business world because the comparability of “creating new markets” and “creating new medical treatments” is limited. While a new treatment may allow treatment to be extended to a larger cohort (e.g., older or sicker patients), the size of the appropriate “market” is still determined primarily by the incidence of the disease. Only when a new treatment allows treatment of a medical condition for which there had been no treatment options can one speak of a disruptive innovation. Thus, VATS lobectomy really only represents a sustaining innovation—a refinement of an existing treatment (perhaps a revolutionary refinement), rather than an entirely new innovation.
What does the future hold? Currently uniportal VATS resection has attracted a great deal of attention. There is certainly a discrepancy between the degree of enthusiasm and the paucity of solid data of benefit over other minimally invasive approaches. Robotic surgery is also gradually increasing—it accounted for approximately 10% of lobectomies in the NIS database in 2013. Again, while it is a viable alternative to VATS, data demonstrating superiority is slim. More novel approaches include subxiphoid approaches and transcervical approaches to lobectomy (49). These have the potential to avoid trauma to the intercostal nerves, but there is limited experience. All of these approaches seem more like further refinements of the approach to lobectomy rather than dramatic deviations. Time will tell what the impact will be.
With respect to surgery, there is a growing appreciation that there are some patients in whom a more limited resection (segmentectomy) is adequate for effective treatment of cancer. More important perhaps is the increasing use of stereotactic body radiotherapy (SBRT). While there is reasonable data that SBRT is a viable alternative for peripheral stage I lung cancers in people who are high risk for surgical intervention, the data seems to indicate that it is slightly less effective than surgery for low risk patients (50). One can speculate about further changes in the treatment of lung cancer: someday resection may be done by alternative imaging techniques that allow blood vessels to be identified without seeing them directly (e.g., ultrasound) or that allow visualization of cancerous vs normal tissues. Further genetic characterization may allow us to differentiate tumors that are localized and lack the ability to metastasize. The innovation that has perhaps the greatest potential to be “disruptive” is CT screening for lung cancer; but this is a complicated interplay of multiple factors (e.g., risk of development of cancer, quality of the scan and interpretation, competing health problems, compliance with the necessary ongoing screening and follow-up of findings, effectiveness of treatment) (51-53). How well this can be implemented broadly is unclear at this time. However, a mere increased prevalence of CT scanning has the potential to significantly change the nature of lung cancer that is brought to a thoracic surgeon’s attention (54).
Conclusions
Both VATS and open lobectomy are well established approaches to lobectomy for lung cancer. A large amount of data demonstrates that VATS results in similar long term survival, a decreased rate of complications and length of stay and a suggestion of a decreased operative mortality—at least in experienced institutions and in appropriately selected patients. The proportion of cases performed by each approach varies by region and by institution, reflecting a multitude of factors. The patient’s body habitus and degree of hilar/mediastinal scarring play a role. The size and location (i.e., large, central) of a tumor affect the appropriateness of VATS. There are clearly tumors and patients in whom VATS is not feasible, although in select cases even sleeve resection and pneumonectomy can be performed by VATS. The availability of the skills and equipment varies, and whether VATS represents a net cost or cost savings varies. Whether it is feasible and worthwhile to overcome the learning curve in one’s setting and patient population will vary from institution to institution. Nevertheless it is clear that both VATS resection and open resection for lung cancer have a role, and should be viewed as complementary rather than competitive approaches. The appropriate degree of uptake of VATS must be defined locally by those caring for patients with lung cancer.
“It always seems impossible until it is done”—Nelson Mandela
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hazelrigg SR, Nunchuck SK, LoCicero J 3rd. Video Assisted Thoracic Surgery Study Group data. Ann Thorac Surg 1993;56:1039-43; discussion 1043-4. [Crossref] [PubMed]
- Roviaro G, Rebuffat C, Varoli F, et al. Thoracoscopy assisted pulmonary lobectomy. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- Walker WS, Carnochan FM, Tin M. Thoracoscopy assisted pulmonary lobectomy. Thorax 1993;48:921-4. [Crossref] [PubMed]
- Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery, Masuda M, Kuwano H, et al. Thoracic and cardiovascular surgery in Japan during 2013: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2015;63:670-701. [Crossref] [PubMed]
- Detterbeck F, Antonicelli A, Okada M. Results of Video Assisted Techniques for Resection of Lung Cancer. In: Pass H, Ball D, Scagliotti G, editors. The IASLC Multidisciplinary Approach to Thoracic Oncology. Aurora, CO: IASLC Press, 2014:385-393.
- Cheng D, Downey RJ, Kernstine K, et al. Video-assisted thoracic surgery in lung cancer resection: a meta-analysis and systematic review of controlled trials. Innovations (Phila) 2007;2:261-92. [Crossref] [PubMed]
- Chen FF, Zhang D, Wang YL, et al. Video-assisted thoracoscopic surgery lobectomy versus open lobectomy in patients with clinical stage I non-small cell lung cancer: a meta-analysis. Eur J Surg Oncol 2013;39:957-63. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Cai YX, Fu XN, Xu QZ, et al. Thoracoscopic lobectomy versus open lobectomy in stage I non-small cell lung cancer: a meta-analysis. PLoS One 2013;8:e82366. [Crossref] [PubMed]
- Paul S, Sedrakyan A, Chiu YL, et al. Outcomes after lobectomy using thoracoscopy vs thoracotomy: a comparative effectiveness analysis utilizing the Nationwide Inpatient Sample database. Eur J Cardiothorac Surg 2013;43:813-7. [Crossref] [PubMed]
- Yang CF, Sun Z, Speicher PJ, et al. Use and Outcomes of Minimally Invasive Lobectomy for Stage I Non-Small Cell Lung Cancer in the National Cancer Data Base. Ann Thorac Surg 2016;101:1037-42. [Crossref] [PubMed]
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: a meta-analysis of propensity score-matched patients. Interact Cardiovasc Thorac Surg 2013;16:244-9. [Crossref] [PubMed]
- Cao C, Zhu ZH, Yan TD, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small-cell lung cancer: a propensity score analysis based on a multi-institutional registry. Eur J Cardiothorac Surg 2013;44:849-54. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010;139:976-81; discussion 981-3. [Crossref] [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [Crossref] [PubMed]
- Stephens N, Rice D, Correa A, et al. Thoracoscopic lobectomy is associated with improved short-term and equivalent oncological outcomes compared with open lobectomy for clinical Stage I non-small-cell lung cancer: a propensity-matched analysis of 963 cases. Eur J Cardiothorac Surg 2014;46:607-13. [Crossref] [PubMed]
- Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009;138:419-25. [Crossref] [PubMed]
- Lee PC, Nasar A, Port JL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2013;96:951-60; discussion 960-1. [Crossref] [PubMed]
- Nwogu CE, D'Cunha J, Pang H, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg 2015;99:399-405. [Crossref] [PubMed]
- Ilonen IK, Räsänen JV, Knuuttila A, et al. Anatomic thoracoscopic lung resection for non-small cell lung cancer in stage I is associated with less morbidity and shorter hospitalization than thoracotomy. Acta Oncol 2011;50:1126-32. [Crossref] [PubMed]
- Murakawa T, Ichinose J, Hino H, et al. Long-term outcomes of open and video-assisted thoracoscopic lung lobectomy for the treatment of early stage non-small cell lung cancer are similar: a propensity-matched study. World J Surg 2015;39:1084-91. [Crossref] [PubMed]
- Jeon JH, Kang CH, Kim HS, et al. Video-assisted thoracoscopic lobectomy in non-small-cell lung cancer patients with chronic obstructive pulmonary disease is associated with lower pulmonary complications than open lobectomy: a propensity score-matched analysis. Eur J Cardiothorac Surg 2014;45:640-5. [Crossref] [PubMed]
- Scott WJ, Matteotti RS, Egleston BL, et al. A comparison of perioperative outcomes of video-assisted thoracic surgical (VATS) lobectomy with open thoracotomy and lobectomy: results of an analysis using propensity score based weighting. Ann Surg Innov Res 2010;4:1. [Crossref] [PubMed]
- Yang CF, Meyerhoff RR, Mayne NR, et al. Long-term survival following open versus thoracoscopic lobectomy after preoperative chemotherapy for non-small cell lung cancer. Eur J Cardiothorac Surg 2016;49:1615-23. [Crossref] [PubMed]
- Cattaneo SM, Park BJ, Wilton AS, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg 2008;85:231-5; discussion 235-6. [Crossref] [PubMed]
- Jones RO, Casali G, Walker WS. Does failed video-assisted lobectomy for lung cancer prejudice immediate and long-term outcomes? Ann Thorac Surg 2008;86:235-9. [Crossref] [PubMed]
- Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: a case-control study. Ann Thorac Surg 1999;68:194-200. [Crossref] [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487-93. [Crossref] [PubMed]
- Farjah F, Wood DE, Mulligan MS, et al. Safety and efficacy of video-assisted versus conventional lung resection for lung cancer. J Thorac Cardiovasc Surg 2009;137:1415-21. [Crossref] [PubMed]
- Park HS, Detterbeck FC, Boffa DJ, et al. Impact of hospital volume of thoracoscopic lobectomy on primary lung cancer outcomes. Ann Thorac Surg 2012;93:372-9. [Crossref] [PubMed]
- Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg 2012;93:1027-32. [Crossref] [PubMed]
- Licht PB, Jørgensen OD, Ladegaard L, et al. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg 2013;96:943-9; discussion 949-50. [Crossref] [PubMed]
- Craig SR, Leaver HA, Yap PL, et al. Acute phase responses following minimal access and conventional thoracic surgery. Eur J Cardiothorac Surg 2001;20:455-63. [Crossref] [PubMed]
- Kirby TJ, Mack MJ, Landreneau RJ, et al. Lobectomy--video-assisted thoracic surgery versus muscle-sparing thoracotomy. A randomized trial. J Thorac Cardiovasc Surg 1995;109:997-1001; discussion 1001-2. [Crossref] [PubMed]
- Zhang Z, Zhang Y, Feng H, et al. Is video-assisted thoracic surgery lobectomy better than thoracotomy for early-stage non-small-cell lung cancer? A systematic review and meta-analysis. Eur J Cardiothorac Surg 2013;44:407-14. [Crossref] [PubMed]
- Taioli E, Lee DS, Lesser M, et al. Long-term survival in video-assisted thoracoscopic lobectomy vs open lobectomy in lung-cancer patients: a meta-analysis. Eur J Cardiothorac Surg 2013;44:591-7. [Crossref] [PubMed]
- Li Z, Liu H, Li L. Video-assisted thoracoscopic surgery versus open lobectomy for stage I lung cancer: A meta-analysis of long-term outcomes. Exp Ther Med 2012;3:886-892. [PubMed]
- Su S, Scott WJ, Allen MS, et al. Patterns of survival and recurrence after surgical treatment of early stage non-small cell lung carcinoma in the ACOSOG Z0030 (ALLIANCE) trial. J Thorac Cardiovasc Surg 2014;147:747-52: Discussion 752-3.
- Berry MF, D'Amico TA, Onaitis MW, et al. Thoracoscopic approach to lobectomy for lung cancer does not compromise oncologic efficacy. Ann Thorac Surg 2014;98:197-202. [Crossref] [PubMed]
- Sugi K, Kaneda Y, Esato K. Video-assisted thoracoscopic lobectomy achieves a satisfactory long-term prognosis in patients with clinical stage IA lung cancer. World J Surg 2000;24:27-30; discussion 30-1. [Crossref] [PubMed]
- Ferguson J, Walker W. Developing a VATS lobectomy programme--can VATS lobectomy be taught? Eur J Cardiothorac Surg 2006;29:806-9. [Crossref] [PubMed]
- Nomori H, Horio H, Naruke T, et al. What is the advantage of a thoracoscopic lobectomy over a limited thoracotomy procedure for lung cancer surgery? Ann Thorac Surg 2001;72:879-84. [Crossref] [PubMed]
- Medbery RL, Gillespie TW, Liu Y, et al. Nodal Upstaging Is More Common with Thoracotomy than with VATS During Lobectomy for Early-Stage Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2016;11:222-33. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Maniscalco LM. A nondivided intercostal muscle flap further reduces pain of thoracotomy: a prospective randomized trial. Ann Thorac Surg 2008;85:1901-6; discussion 1906-7.
- Wu N, Yan S, Wang X, et al. A prospective, single-blind randomised study on the effect of intercostal nerve protection on early post-thoracotomy pain relief. Eur J Cardiothorac Surg 2010;37:840-5. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Detterbeck FC, Kim AW, Zielinski M. Looking in from above and up from below: new vistas in thoracic surgery. Innovations (Phila) 2012;7:161-4. [Crossref] [PubMed]
- Rosen JE, Salazar MC, Wang Z, et al. Lobectomy versus stereotactic body radiotherapy in healthy patients with stage I lung cancer. J Thorac Cardiovasc Surg 2016;152:44-54.e9. [Crossref] [PubMed]
- Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012;307:2418-29. [Crossref] [PubMed]
- Ahmad U, Detterbeck FC. Current status of lung cancer screening. Semin Thorac Cardiovasc Surg 2012;24:27-36. [Crossref] [PubMed]
- Mazzone P, Powell CA, Arenberg D, et al. Components necessary for high-quality lung cancer screening: American College of Chest Physicians and American Thoracic Society Policy Statement. Chest 2015;147:295-303. [Crossref] [PubMed]
- Detterbeck FC. Maintaining aim at a moving target. J Thorac Oncol 2011;6:417-22. [Crossref] [PubMed]
Cite this article as: Detterbeck F, Molins L. Video-assisted thoracic surgery and open chest surgery in lung cancer treatment: present and future. J Vis Surg 2016;2:173.


