Thoracoscopic anatomic segmentectomies for lung cancer: technical aspects
Although results of trials comparing sublobar to lobar resections for lung cancer are still pending, interest for thoracoscopic sublobar resections (TSLR) is growing because of the increased incidence of clinical stage I non-small cell lung carcinoma (NSCLC) and ground glass opacities (GGO). Indeed, several studies have demonstrated that the postoperative morbidity is less after SLR than after lobectomy (1,2) and the thoracoscopic approach has a better outcome than thoracotomy, especially with regard to pulmonary complications (3,4). Some publications report a satisfactory overall survival (OS) (3,5,6) whereas other report a better OS after lobectomy (7). Until now and while waiting for the results of ongoing trials, lobectomy remains the standard treatment (8). If applied to early stages of lung cancer, thoracoscopic segmentectomies must be performed according to some oncological principles as defined by the International Association for the Study of Lung cancer (IASLC): free resection margins proved microscopically, systematic nodal dissection or lobe-specific systematic nodal dissection, no extracapsular nodal extension of the tumor; and the highest mediastinal node removed must be negative (9). The aim of this article is to describe our technique, basing on our experience of 235 TSLR for pulmonary malignancy. We will not deal with technical details that are not directly related to cancer indication. Whatever the approach—multiple or single ports—most of the technical points hereby described should be valid.
Experience
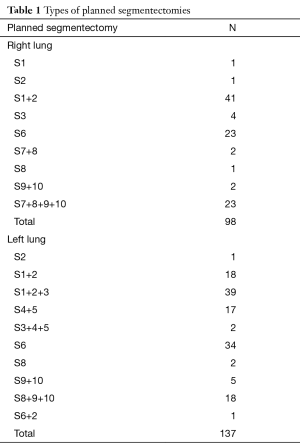
From January 2007 to July 2016, 235 thoracoscopic anatomical segmentectomies have been performed in 232 patients. Indication for segmentectomy was a proven or suspected NSCLC in 184 and metastasis in 51. Patients had both oral and written information about the procedure. Intraoperative and postoperative data were recorded in a prospective manner in a database that was approved from our Institutional Review Board (CEPAR 2013-002). Four patients had two procedures, either for synchronous bilateral metastasis (three patients) or for two metachronous NSCLC (one patient). The types of segmentectomy are summarized in Table 1.
Full table
Segmentectomies are performed according to a previously reported technique based on a full thoracoscopic and fissure-first approach (10-12).
There was 1 intraoperative death whose case has been reported (13). In the remaining 234 surgical procedures median operation time was 150 min (range, 50−332 min). The median estimated blood loss was 60 mL [ranged from 0 (non-measurable) to 900 mL].
There were eight conversions into a posterolateral thoracotomy (3.4%). Among these, an unplanned additional resection was performed in one patient (a S1+2 segmentectomy transformed into an upper lobectomy because of a vascular injury), while in the seven other patients; the initially planned segmentectomy was completed. One additional patient had a secondary thoracotomy at day 5 for a lingular ischemia after a trisegmentectomy.
Ten other patients had an intraoperative unplanned resection (eight lobectomy and two basilar resections) that did not require thoracotomy and another patient had a lingular ischemia after a left trisegmentectomy that required a thoracoscopic lingulectomy at D2.
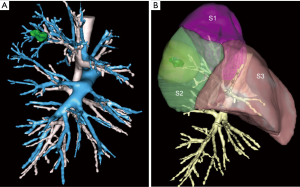
With respect to the ten additional resections, seven were for oncological reasons (3%). In one patient, an intersegmental lymph node was invaded at frozen section (Figure 1) and in six patients the parenchymal section line was too close to the tumor to be considered as free resection margins.
Postoperative mortality rate was 0.8%. One patient died from an acute respiratory distress syndrome and a second one from acute proximal pulmonary embolism. All but 37 patients had an uneventful postoperative course (15.7%). The two cases of postoperative lingular ischemia required a reoperation for completion lingulectomy, i.e., these patients had finally a left upper lobectomy. The median drainage duration was 3 days (range, 1–13 days) and the median hospital stay was 5 days (range, 2–21 days).
Preoperative modelisation
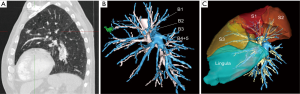
Several teams have demonstrated the study of preoperative computed tomography (CT) three-dimensional reconstruction helps asserting the number and direction of arteries with good correlation (14-16). With the vascular pattern in mind the surgeon can perform a safer dissection of the pulmonary artery branches, especially when the fissure is fused and/or when lymph nodes are present. In the first phase of our experience, patients selected for a segmentectomy had a multidetector row preoperative CT angiography with three-dimensional volume-rendering reconstruction of arterial and venous anatomy. This method had several drawbacks: (I) it was time consuming for the radiologist; (II) there was no possibility to study the anatomy by focusing on a single element, e.g., venous or arterial pattern; and (III) the surgeon could not manipulate the model and/or fuse broncho-vascular elements.
Currently, we use an online service that provides patient’s 3D modeling from medical DICOM images (Visible Patient™, Strasbourg, France). The images are anonymized and then uploaded by the surgeon on a secured dedicated web portal. A 3D model from these images is created according to the surgeon’s needs. The 3D model can be used via a Virtual Planning software and manipulated from any direction or any point of reference, either on a laptop, on a tablet or smartphone. All members of the surgical team have a direct access to the images on their own phone or tablet so that strategy and technical issues can be discussed within the group at any time. The software not only allows studying the main anatomical landmarks, in total or separately, but provides the following helpful functions:
- Display of virtual resections by clicking on a selected bronchus;
- Calculation of segment volumetry;
- Calculation and simulation of a safety margin, actually twice the larger diameter of the tumor, which is represented by a yellow halo around the tumor. This helps visualizing if the foreseen segmentectomy will respect a security margin, or if a more extended resection must be preferred.
Most patients whose renal function is compatible with an injected CT-scan have a preoperative modeling. Some patients don’t have this reconstruction, either because they cannot afford an injected CT-scan or because the surgeon is reluctant asking for an injected CT if the patient has already undergone a non-injected CT just before the operation.
Figures 1 and 2 demonstrate two situations where simulation helps choosing the appropriate treatment: Figure 1 demonstrates a typical example where a right posterior segmentectomy has been foreseen (Figure 1A). Three-dimensional reconstruction shows the safety margin was widely sufficient (Figure 1B). Figure 2 illustrates the case of a patient with very limited pulmonary function and comorbidities. The small nodule visible on CT was a squamous cell carcinoma, proven by percutaneous biopsy under CT guidance. On CT, this nodule looked like being located in segment S3 and an anterior segmentectomy was foreseen (Figure 2A). However, preopeorative modelisation demonstrates the nodule was actually not in S3 but in S1 (Figure 2B). By adding a safety margin, it can be seen that a bisegmentectomy S3 + S1 had to be performed (Figure 2C). Surgery was abandoned and the patient was finally referred for stereotactic body radiation therapy (SBRT).
Instrumentation
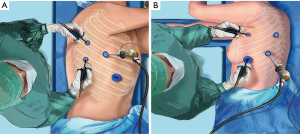
As for thoracoscopic lobectomies, only dedicated thoracoscopic instruments are used (Delacroix-Chevalier©, Paris, France). These are inserted through three to four trocars (Figure 3). Depending on the type of inserted instruments, their diameter is comprised between 3 and 25 mm. The control of large vessels is accomplished with endostaplers while hemostasis of small caliber vessels is performed with clips or with a bipolar vessel sealing device (Enseal™, Ethicon) or with a combination of both. The intersegmental plane is divided by a combination of bipolar sealing device (for its peripheral and thin portion) and stapling (for its central and thick portion) (Endo-GIA™, Medtronic). As for oncological needs, we have developed special instruments facilitating exposure of station 7 (Figure 4) and lymph node grasping forceps whose aim is avoiding, or at least, minimizing crushing nodes during dissection. As manual suturing of the bronchial stump can be necessary, e.g., for a tumor at the origin of a segmental bronchus (Figure 5), a suture kit and the corresponding skills must be available in the OR.


Imaging system
We use a deflectable scope housing a distal CCD (LTF™, Olympus©, Tokyo, Japan) connected to a high definition camera system (HDTV) (9). The new generation scopes have a heating feature that efficiently prevents fogging. In addition, we have developed a specially designed trocar that deflects blood drops from the scope’s tip, so that we can keep a clear image throughout the procedure. The endoscope is held by a mechanical (Olympus SH-1) or robotized (Viky®, EndocontrolTM) scope holder and the image is displayed on three monitors.
General technical aspects (Figure 6)

The procedure is performed under general anesthesia with split ventilation using a double-lumen endotracheal tube. Patients are positioned in lateral decubitus as for a thoracotomy. The surgeon stands at the back of the patient for right side resections and on the front for left resections (Figure 7). For patients operated on for lung cancer, intersegmental lymph nodes are analyzed by frozen section to confirm the indication of segmentectomy (10). Demarcation between the resected and preserved segments is usually made possible by gentle reventilation and adequate application of a long 5-mm lung forceps whose position is adapted according to the inflation-deflation line. When the remaining segment is mobile and at risk of torsion, it is anchored to the adjacent lobe by stitches or by stapling using a TA endostapler. An additional radical lymphadenectomy is performed for all patients operated on for a suspicion of primary NSCLC. No utility incision is used. On completion of the segmentectomy, the specimen is wrapped into an endobag and retrieved through one of the port sites that are enlarged to a length of 2 to 4 cm, depending on the specimen size. The use of a rib spreader is never required for this task. For patients operated on for NSCLC, intraoperative pathological examination is done on the portion of the staple line facing the tumor if the latter is at less than 2 cm from the resection margin. In most cases, only one chest tube is placed through one of the port site.
Conversions
There were eight intraoperative conversions into thoracotomy in our series (3.4%). Apart of the recent study of Witte et al. reporting a 30% conversion rate (20), switches to thoracotomy are rare in most series, ranging from 0% to 5%. A 0% rate seems surprising and one may wonder whether there could be a bias related to a non-intention-to-treat analysis in some series.
In our series 5 out of the 8 conversions were related to a vascular (n=4) or parenchymal injury (n=1). There was only one conversion for oncological reason in which the nodule could not be found during the exploration step, leading to a manual palpation by thoracotomy. This stresses the importance of a preoperative localization technique or a preoperative biopsy, whatever the method (hook wire or dye marking under CT scan guidance or endobronchial electronavigation).
Margins
Compared to lobectomies, SLR comprise an increased risk of local recurrence as resection margins tend being closer to the tumor. Schuchert et al. have clearly demonstrated that segmentectomies outcomes compare favorably with lobectomy for stage I NSCLC but that margin/tumor ratios of less than one are associated with a higher rate of recurrence (21). When the margin/tumor diameter ratios exceeded one, it was associated with a significant reduction in recurrence rates compared with ratios of less than 1 (25.0% vs. 6.2%; P=0.0014). In their study, the rate of local recurrence was 7.7% and was correlated in most cases with a tumor margin less than 2 cm. In our series, the margin was insufficient in six patients leading to extend the segmentectomy to the adjacent segment or to the lobe. It should be noticed that a borderline margin sometimes came as a surprise to the surgeon who thought section was widely distant from the tumor. Preoperative modeling can help making decision and plan the adequate TSLR. On the Visible Patient™ software, preoperative visualization of the safety margin can help avoiding several unplanned extensive resections.
Lymph nodes
As shown on Figure 8, lymph nodes are frequently encountered at the origin of a segmental bronchus. These nodes must be cleared and analyzed during the procedure. Would the nodes be invaded at frozen section, the procedure should be transformed into a lobectomy. There are consistent data in the literature suggesting intersegmental lymph node clearance to be of major importance during thoracoscopic segmentectomies (8,23). Wolf et al. have demonstrated that in small NSCLC, lobectomy was associated with longer overall and recurrence-free survival. Sublobar resections were associated with an increased rate of local recurrence (16% vs. 8%, P=0.1117). However, when lymph nodes were sampled during segmentectomy, local recurrence rate and overall and recurrence-free survival distributions were similar to those for lobectomy (23). In the beginning of our experience, we were clearing all intersegmental lymph nodes but asked for frozen section only when these looked suspicious. However, since we have discovered neoplastic cells in small intersegmental lymph nodes that looked macroscopically benign, we now always ask for an intraoperative pathological analysis. Swanson has suggested checking a representative intersegmental or “sump” node and convert to a lobectomy if the latter is positive (24). However deciding which one is the sump node—which besides can be negative on Pet-scan—is not obvious and could be a research topic aiming at determining a sentinel node (25). At that time we do prefer analyzing all intersegmental lymph nodes during the procedure.

Localization error
In one patient, the nodule could not be localized and a thoracotomy was necessary for digital palpation. This is not a specific issue of SLR as such a failure can be faced during any video-assisted procedure. In this patient, a preoperative localization technique could have avoided a thoracotomy. However, marking the nodule is only helpful if the target is not too far away from the pleural surface. Marking can be done either with methylene blue labeling or by a hook-wire placement under CT-scan guidance or can be bronchoscopically achieved (26) depending on surgeon’s preference and available technology. But, for all deeply located nodules, preoperative marking does not help much because it does not ease resection which is limited by the tissue thickness and insufficient safety margin. In these situations a primary anatomical segmentectomy can be a better option than a wedge resection, provided the target nodule is in the resected segment.
The three other localization errors raise more concerns. Despite the use of a preoperative modelisation of bronchovascular structures in 2 of these 3 patients, the target nodule was not found on the specimen and forced us to extend the resection to the adjacent segment. To our knowledge, this issue has not yet been reported. However surgeons experienced with TSLR are aware of this mistake (26). Even though the target nodule is localized on preoperative mapping and the anatomy is reconstructed, spatial disorientation during the procedure is possible because the lung collapse induces changes in landmarks. This is particularly valid for basilar segments in which stapling does not follow a 2-dimensional pattern but a 3-dimensional one. In these cases, determining the precise boundaries of the segments to be resected is essential. This can be achieved by the so-called virtual assisted lung mapping described by Sato et al. (26) where a multispot dye-marking via bronchoscopy is used for determination of the resections margins. The alternate method consists in the coloration of the whole segment(s) to be resected by intrabronchial injection of methylene blue (27) or indocyanine green (ICG) (28) or systemic injection of ICG using an near infrared imaging system (29). All these methods have their advantages and shortcomings. Finding the best compromise should be one of our priorities in the forthcoming years.
Conclusions
Even though the study of registers and ongoing trials may come to the conclusion that lobar resections have a slightly better OS than sublobar resections, the fact remains that (I) many of our patients cannot afford a lobectomy, especially those who have already undergone a lobectomy for a previous lung cancer; (II) many GGO and metastasis cannot be satisfactory treated by a mere wedge resection and require an anatomic segmentectomy. As it is now clear that a segmentectomy has a much better outcome when done by thoracoscopy rather than by thoracotomy (4), we have to do our best make TSLR a precise, safe and oncological procedure that competes favorably with alternate and less satisfactory treatments such as SBRT or radiofrequency. This is one of the challenges of the forthcoming years that will require not only technical skills but also the use of outstanding technologies such as modelisation, preoperative planning and mapping.
Acknowledgements
Three-dimensional modelisations used in this study were made possible thanks to a grant from the Ligue contre le Cancer.
Footnote
Conflicts of Interest: One of the authors (DG) is consultant for an instrument manufacturer (Delacroix Chevalier). The other authors have no conflict of interest to declare.
References
- Kim SJ, Ahn S, Lee YJ, et al. Factors associated with preserved pulmonary function in non-small-cell lung cancer patients after video-assisted thoracic surgery. Eur J Cardiothorac Surg 2016;49:1084-90. [Crossref] [PubMed]
- Deng B, Cassivi SD, de Andrade M, et al. Clinical outcomes and changes in lung function after segmentectomy versus lobectomy for lung cancer cases. J Thorac Cardiovasc Surg 2014;148:1186-1192.e3. [Crossref] [PubMed]
- Ghaly G, Kamel M, Nasar A, et al. Video-Assisted Thoracoscopic Surgery Is a Safe and Effective Alternative to Thoracotomy for Anatomical Segmentectomy in Patients With Clinical Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:465-72; discussion 472. [Crossref] [PubMed]
- Traibi A, Grigoroiu M, Boulitrop C, et al. Predictive factors for complications of anatomical pulmonary segmentectomies. Interact Cardiovasc Thorac Surg 2013;17:838-44. [Crossref] [PubMed]
- Cao C, Gupta S, Chandrakumar D, et al. Meta-analysis of intentional sublobar resections versus lobectomy for early stage non-small cell lung cancer. Ann Cardiothorac Surg 2014;3:134-41. [PubMed]
- Schuchert MJ, Abbas G, Awais O, et al. Anatomic segmentectomy for the solitary pulmonary nodule and early-stage lung cancer. Ann Thorac Surg 2012;93:1780-5; discussion 1786-7.
- Khullar OV, Liu Y, Gillespie T, et al. Survival After Sublobar Resection versus Lobectomy for Clinical Stage IA Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2015;10:1625-33. [Crossref] [PubMed]
- Whitson BA, Groth SS, Andrade RS, et al. Survival after lobectomy versus segmentectomy for stage I non-small cell lung cancer: a population-based analysis. Ann Thorac Surg 2011;92:1943-50. [Crossref] [PubMed]
- Rami-Porta R, Wittekind C, Goldstraw P, et al. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33. [Crossref] [PubMed]
- Gossot D, Zaimi R, Fournel L, et al. Totally thoracoscopic pulmonary anatomic segmentectomies: technical considerations. J Thorac Dis 2013;5 Suppl 3:S200-6. [PubMed]
- Gossot D. Totally thoracoscopic basilar segmentectomy. Semin Thorac Cardiovasc Surg 2011;23:67-72. [Crossref] [PubMed]
- Gossot D. Left lower lobe: basal segments. In: Gossot D. Atlas of endoscopic major pulmonary resections. Paris: Springer-Verlag, 2010:170.
- Decaluwe H, Petersen RH, Hansen H, et al. Major intraoperative complications during video-assisted thoracoscopic anatomical lung resections: an intention-to-treat analysis. Eur J Cardiothorac Surg 2015;48:588-98; discussion 599. [Crossref] [PubMed]
- Chen-Yoshikawa TF, Date H. Update on three-dimensional image reconstruction for preoperative simulation in thoracic surgery. J Thorac Dis 2016;8:S295-301. [PubMed]
- Hagiwara M, Shimada Y, Kato Y, et al. High-quality 3-dimensional image simulation for pulmonary lobectomy and segmentectomy: results of preoperative assessment of pulmonary vessels and short-term surgical outcomes in consecutive patients undergoing video-assisted thoracic surgery†. Eur J Cardiothorac Surg 2014;46:e120-6. [Crossref] [PubMed]
- Kanzaki M, Kikkawa T, Shimizu T, et al. Presurgical planning using a three-dimensional pulmonary model of the actual anatomy of patient with primary lung cancer. Thorac Cardiovasc Surg 2013;61:144-50. [Crossref] [PubMed]
- Gossot D, Lutz J, Grigoroiu M, et al. Use of 3-legs retractor for exposure of station seven on the left side to ease lymph node dissection. Asvide 2016;3:457. Available online: http://www.asvide.com/articles/1231
- Gossot D, Lutz J, Grigoroiu M, et al. Example of manual division of the bronchus during a left lingula-sparing upper lobectomy for carcinoid tumor. At frozen section, the bronchial margin was not free and was then cut back. Asvide 2016;3:458. Available online: http://www.asvide.com/articles/1232
- Gossot D, Lutz J, Grigoroiu M, et al. Example of a full thoracoscopic approach for anatomic segmentectomy. In this case, a left S9+10 segmentectomy for a ground glass opacity. Asvide 2016;3:459. Available online: http://www.asvide.com/articles/1233
- Witte B, Stenz C, Vahl CF, et al. Comparative intention-to-treat analysis of the video-assisted thoracoscopic surgery approach to pulmonary segmentectomy for lung carcinoma‡. Interact Cardiovasc Thorac Surg 2015;21:276-83. [Crossref] [PubMed]
- Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:926-32; discussion 932-3. [Crossref] [PubMed]
- Gossot D, Lutz J, Grigoroiu M, et al. Example of lymph nodes clearance at the bifurcation of B3 and B1+2 during a right S3 segmentectomy for cT1aN0 NSCLC. If invaded at frozen section, the procedure must be converted into an upper lobectomy. Asvide 2016;3:460. Available online: http://www.asvide.com/articles/1234
- Wolf AS, Richards WG, Jaklitsch MT, et al. Lobectomy versus sublobar resection for small (2 cm or less) non-small cell lung cancers. Ann Thorac Surg 2011;92:1819-23; discussion 1824-5.
- Swanson SJ. Video-assisted thoracic surgery segmentectomy: the future of surgery for lung cancer? Ann Thorac Surg 2010;89:S2096-7. [Crossref] [PubMed]
- Fukutomi T, Kohno M, Izumi Y, et al. Sentinel node microscopic metastasis detected after segmentectomy for lung cancer followed by completion lobectomy: two case reports. Thorac Cardiovasc Surg 2012;60:421-4. [PubMed]
- Sato M, Yamada T, Menju T, et al. Virtual-assisted lung mapping: outcome of 100 consecutive cases in a single institute. Eur J Cardiothorac Surg 2015;47:e131-9. [Crossref] [PubMed]
- Zhang Z, Liao Y, Ai B, et al. Methylene blue staining: a new technique for identifying intersegmental planes in anatomic segmentectomy. Ann Thorac Surg 2015;99:238-42. [Crossref] [PubMed]
- Sekine Y, Ko E, Oishi H, et al. A simple and effective technique for identification of intersegmental planes by infrared thoracoscopy after transbronchial injection of indocyanine green. J Thorac Cardiovasc Surg 2012;143:1330-5. [Crossref] [PubMed]
- Kuroda H, Dejima H, Mizumo T, et al. A new LigaSure technique for the formation of segmental plane by intravenous indocyanine green fluorescence during thoracoscopic anatomical segmentectomy. J Thorac Dis 2016;8:1210-6. [Crossref] [PubMed]
Cite this article as: Gossot D, Lutz J, Grigoroiu M, Brian E, Seguin-Givelet A. Thoracoscopic anatomic segmentectomies for lung cancer: technical aspects. J Vis Surg 2016;2:171.