Evolution of a minimally invasive mitral valve program
Introduction
Since the very first beginning of port access surgery in the mid of 1990s, video assistance evolved to an important tool in mitral valve surgery (1). The minimally invasive access advanced from partial sternotomy to anterolateral minithoracotomy and from direct vision via endoscopic guidance to totally endoscopic procedures (2-4).
Over the last years minimally invasive surgery evolved to a highly accepted, safe and effective treatment in mitral valve disease. Mitral valve repair being the gold standard in primary mitral regurgitation (MR) is ideally performed by experienced surgeons in centers with a high patient volume (5).
The procedure requires a sophisticated set up and works best in an environment of collaboration between skilled team members and heart surgeons. Besides the smaller incision, the mini-thoracotomy permits a direct view to the mitral valve and the assessment of the pathology, which counts as the biggest advantage compared to open surgery. Even complex mitral valve repairs are feasible with a clear view of the leaflets, the subvalvular apparatus, papillary muscles and the interior of the left ventricle (LV).
The improvement of many technical features over the last years allow for a safe performance and achievement of reliable high quality results. In this context the intra-aortic balloon clamping and the use of a full HD 3D system are milestones in the evolution of minimally invasive mitral surgery.
Patient selection and work up
To become familiar with the minimally invasive technique and access it is important to master the first 30 (at least) cases smoothly. Patient selection is the first big step to prevent complications. Experience has shown that repeated occurrence of intraoperative problems with concurrent longer OR times often leads to abandoning the procedure.
In our present days obesity is a growing problem. Patients with a normal body mass index (BMI) should be selected for the very first cases. The ideal patient is tall with a low BMI. The body habitus and the important level of the diaphragm can easily be identified in the chest X-ray.
“Rather get in higher than be too low!”
The level of the intercostal incision (we prefer a skin incision lateral to the mammilla and in women in the submammarian fold) should be in a safe distance from the diaphragm. We mainly use the 4th intercostal space as an access to the heart. A lowered hemidiaphragm allows for an uncompromised view and working angle. By elevation the general view should be improved with a surgical technique.
Valve pathology is another important issue to consider. Complex diseased valves, such as bileaflet or bicommissural prolaps or even Barlow valves as well as redo scenarios should be avoided in the beginning. Starting with a Type I ring annuloplasty is a good way to create confidence. When gaining expertise more complex repairs and concomitant procedures such as tricuspid valve reconstruction or atrial fibrillation (AF)—cryoablation can be performed.
For the evaluation of the underlying pathology, echocardiography is the method of choice. Knowing the cause of MR enables to plan the repair strategy. Transesophageal echo should be performed perioperatively for baseline information such as height of the anterior mitral leaflet, size of annulus and left atrium (LA) and exclusion of annular or leaflet calcifications, thrombus and aortic regurgitation (AR). Arresting the heart in presence of mild AR is acceptable but adequate myocardial protection and decompression of the LV should be considered.
Treatment of patients with a severe impaired left ventricular function remains difficult, not only because of the low ejection fraction. The valvular apparatus appears normal but tethering of the leaflets results in a restriction of leaflet motion due to LV dilatation.
Pre-operative preparation
The clarification of the coronary status is of course essential and should be performed within 6 months preoperatively. In younger patients without a family history of coronary artery disease a cardiac CT-scan may be considered. In single vessel disease, which means a significant but not critical stenosis, a hybrid approach is an option to avoid open surgery, in which the PCI should follow the valve repair because of the double platelet anticoagulation.
Peripheral vessel condition ought to be clarified in nearly every patient with or without known peripheral artery disease (PAD). The use of CT scan data delivers detailed information about the status of peripheral vessels and the diameter and condition of the aorta. We use the intra-aortic balloon technique routinely in patients with an aortic diameter not greater than 40 (max.42) mm instead of external cross clamping. Therefore the evaluation of the ascending aorta is important to achieve a sufficient and consistent occlusion.
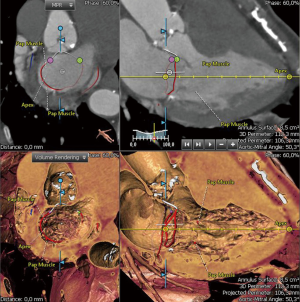
Postprocessing of CT data and the use of the 3mensio software (ANGIO CONSULT, Speyer, Germany) for instance provides exact data of the left heart. Besides measuring the anatomical annulus, all subvalvular structures are assessable and may help to plan the surgical strategy (Figure 1). The volumetric measurement of the LA is clearly underestimated in 2D echo as well as 3D echo and more precise by using CT or MRI data. The LA volume index may help to predict the onset of AF.
In the presence of AF endocardial cryoablation (MAZE procedure) and closure of the left atrial appendage (LAA) should be scheduled as concomitant procedures. With the minimally invasive access the options for reducing the risk of stroke are either exclusion by endocardial suturing with a double layer 4.0 Prolene or epicardial clipping of the LAA. Several studies have shown a successful closure rate between 40% and 50%. The latest and from our point of view better option is the use of the Atriclip® (AtriCure) (CE-marked and FDA approved) through the transverse sinus. Because of its repositioning option the clip can be placed safely and highly effective under TEE control.
Equipment preference card
- Long shafted MIVS instruments;
- Mechanical and pneumatic arm (Unitrac, Aesculap, Germany);
- Intra-aortic Balloon (Intraclude, Edwards Lifesience, USA);
- Endoreturn cannula (Edwards Lifesience, USA);
- Two stage venous cannula (Sorin, Italy);
- Full HD 3D camera and monitor (Einstein vision, Aesculap, Germany);
- Soft Tissue Retractor (Geister, Germany);
- Atrial roof retractor (Fehling, Germany);
- NIRS (Near Infrared Reflectance Spectroscopy).
Procedure and the role of team members
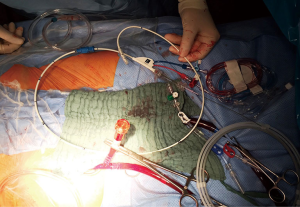
In a routine patient the procedure starts with cannulation of the right femoral vessels to establish cardiopulmonary bypass. The arterial cannula consists of an additional side arm for balloon entry and is available in 21 and 23 F. Figure 2 shows the Balloon system which will be placed in the ascending aorta. Aortic dilatation is acceptable up to 40 mm; otherwise the maximum filling volume of 35 cc is not sufficient enough to occlude the aortic lumen.
The use of an endovascular clamping system allows for antegrade delivery of crystalloid cardioplegia, root venting and for deairing by the end of the procedure. Changing the aortic clamping from Chitwood—towards the Balloon-technique was a milestone in our institution and a big forward step to the total endoscopic approach. Why? There are a few steps less to do. The application of cardioplegia via needle vent direct into the aorta had led to a number of unnecessary bleeding problems on the ascending aorta. Additionally, aortic clamping includes the potential risk of damaging the aortic wall, pulmonary artery or LAA and the occurrence of emboli. Now the surgeon can focus on the LA and the mitral valve problem. Because of the smaller working angle a rib retraction is not necessary anymore.
The establishment of cardiopulmonary arrest is a team approach. So, the scrub nurse is responsible for the preparation of the balloon system, which includes deairing and flushing of all lumina.
The Anesthetist is responsible for the intraoperative echo, balloon position, bilateral radial arterial blood pressure and aortic root pressure control. After introducing the femoral guide wire, echocardiographic identification is necessary inside the descending followed by the ascending aorta. Loss of left or right radial artery pressure after balloon filling indicates a dislocation (migration) of the balloon and requires correction. Reaching the exact position between aortic valve and right truncus the perfusionist as well as the surgical assistant is monitoring the aortic balloon inflation pressure and arterial perfusion pressure. Balloon pressure should not be used as an only indicator for occlusion.
The assistant should manage the filling status of the balloon during the whole operation.
The setup for hemodynamic monitoring should show an overlap of right and left radial artery (different colors), aortic root pressure and CVP. Monitors must be visible to surgeons, anesthetists and perfusionists (Figure 3)!

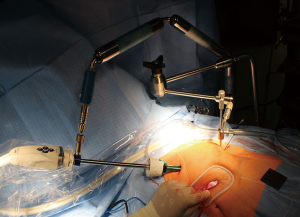
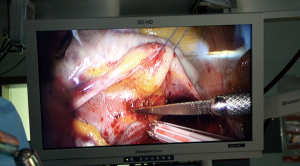
For visualization, the endoscopic 3D HD Camera (EinsteinVision 2.0, Aesculap, Germany) is introduced using a 10 mm standard port through the chest wall right above the incision and held in position through a pneumatic arm (Unitrac device, Aesculap, Germany) (Figures 4,5). All further steps are carried out video directed while wearing 3D polarization glasses. The excellent depth view allows easier orientation while performing surgery. Of course it seems strange to look at the screen when your hands are doing things in a different place. Very often it is more challenging to work under direct vision especially in obese patients with an elevated diaphragm. In these cases a U-stitch along the right diaphragmatic dome improves the general view (Figure 3).
Modern technical features are very convincing, so the camera comes with an anti-fog technology on board. A “heater” is integrated into the distal tip of the endoscope to balance out the differences in temperatures and provides a clear view throughout the whole procedure. The autofocus effect ensures that the entire surgical field is always in focus. The latest edition facilitates a digital zoom with three zoom settings (1.3, 1.6 and 1.9) to optimize the image size. Aesculap uses native-resolution full HD sensors in 3D endoscopy, which provide a low-noise, high-contrast, razor-sharp image in 16:9 format. A sterile single use sheath is used and seals the endoscope in the operation field. This sterile concept enables continuous availability of the camera system (7).
The optical system is available with a zero- or a thirty-degree dual channel endoscope. Staying close to our incision with the camera port we always stick on the zero-degree scope mimicking direct vision on the screen.
Post-operative management
Straight forward mitral valve repair cases have a short ventilation time and are good candidates for a fast track regime. The implementation of this concept requires a meticulous surgical work with optimal results and a low postoperative bleeding incidence. The anesthetists should use a balanced analgesic and sedative medication. The use of an intercostal catheter allows for pain control up to 2 or 3 days without any movement restrictions. All invasive structures such as chest tubes or IV-lines are intended for fast removal.
Tips, tricks and pitfalls
- Preoperative CT scan to screen for pad (plaques/vessel abnormalities);
- Creation of a 3D model to determine size of LA, LV and annulus as well as papillary muscle distribution;
- If retrograde perfusion seems too risky consider cannulation of axillary artery or direct aortic cannulation;
- Ensure position of venous cannula via TEE;
- In case of severe adhesions convert to sternotomy;
- Elevated diaphragm can be lowered (pulled down) by a U-stich to improve vision and intrathoracic space;
- Save the phrenic nerve!
Weaning of cardiopulmonary bypass: use TEE for screening for complications:
- Identification of CX and its perfusion;
- Check for complete deairing of the LV.
Postbypass:
- Localize and quantify residual MR;
- Examine for mitral stenosis;
- Screen for global and regional ventricular dysfunctions;
- Look for SAM;
- Exclude new AR.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Carpentier A, Loulmet D, Le Bret E, et al. Open heart operation under videosurgery and minithoracotomy. First case (mitral valvuloplasty) operated with success. C R Acad Sci III 1996;319:219-23. [PubMed]
- Mohr FW, Falk V, Diegeler A, et al. Minimally invasive port-access mitral valve surgery. J Thorac Cardiovasc Surg 1998;115:567-74; discussion 574-6. [Crossref] [PubMed]
- Vanermen H, Farhat F, Wellens F, et al. Minimally invasive video-assisted mitral valve surgery: from Port-Access towards a totally endoscopic procedure. J Card Surg 2000;15:51-60. [Crossref] [PubMed]
- Casselman FP, Van Slycke S, Wellens F, et al. Mitral valve surgery can now routinely be performed endoscopically. Circulatio 2003;108 Suppl 1:II48-54. [Crossref] [PubMed]
- Seeburger J, Borger MA, Falk V, et al. Minimal invasive mitral valve repair for mitral regurgitation: results of 1339 consecutive patients. Eur J Cardiothorac Surg 2008;34:760-5. [Crossref] [PubMed]
- Czesla M, Mogilansky C, Balan R, et al. Minimally invasive mitral valve repair using intra-aortic balloon occlusion and 3D HD camera system. Asvide 2016;3:455. Available online: http://www.asvide.com/articles/1228
- Aesculap product brochure (No. C92602 0214/0,75/1). Available online: https://www.aesculap.extranet.bbraun.com/public/frame_doc_index.html?med_id=1000151956
Cite this article as: Czesla M, Mogilansky C, Balan R, Kattner S, van Ingen G, Massoudy P. Evolution of a minimally invasive mitral valve program. J Vis Surg 2016;2:169.