Robotically-assisted mitral valve repair—chordal replacement with Gore-Tex loops
Highlight box
Surgical highlights
• The loop technique is as equally applicable to robotic surgery as it is to video-assisted ‘mini mitral’ surgery.
What is conventional and what is novel/modified?
• Gore-Tex loops, as pioneered by Mohr, are widely used in Europe for video-assisted ‘mini mitral’ surgery and have proven durability, but their use in robotic surgery has not been widely reported.
• A modification to the technique of loop length measurement during robotic valve reconstruction is helpful.
• Fixed length loops can easily have their ‘effective length’ varied if the initial measurement is not optimal in the completed repair.
What is the implication, and what should change now?
• The loop technique simplifies the repair of complex multi-segment prolapse, and its wider adoption during robotic surgery will allow more patients with increasingly complex valves to benefit from totally endoscopic valve reconstruction.
Introduction
Background
Since the seminal work of Drs. Frater and David (1,2), the use of Gore-Tex neochords for mitral valve repair is now routine and has excellent long-term outcomes (3). They increase the likelihood of a successful mitral valve repair and are associated with a lower reoperation rate and favourable valve haemodynamics (lower transmitral gradient and larger orifice area) when compared with leaflet resection (4). Accurate judgement of length against a non-prolapsing reference point is critical but as expanded polytetrafluoroethylene (ePTFE) is slippery, knots can slide during tying, and this is more likely to happen when using a knot pusher during minimally invasive surgery.
In 2000, Mohr devised a method of creating pre-measured loops of ePTFE, which negated the risk of inadvertently shortening the chordal length during tying (5). The initial paper reported this technique in 10 consecutive patients with anterior leaflet prolapse, with a satisfactory repair in all and no need for alteration of chordal height after insertion. Half of the patients were operated on using a minimal access approach with video-assistance.
In 2008, the Leipzig group published two important manuscripts about their loop technique. They reported on 632 patients undergoing mitral valve repair with Gore-Tex loops, 83% via mini-thoracotomy, across a variety of leaflet prolapse subsets (posterior 49%, anterior 24%, bileaflet 27%) (6). Only 4% of patients had >1+ mitral regurgitation at discharge with 97.4%±1.4% freedom from reoperation at 3 years post-operatively. A prospective randomised trial of loops vs. leaflet resection in 129 patients undergoing minimally invasive mitral valve repair revealed a greater depth of coaptation after implantation of loops (7.6±3.6 vs. 5.9±2.6 mm, P=0.03) but no difference in orifice area or gradients (7).
The largest published experience to date, again from Leipzig, was in 2,134 consecutive patients undergoing minimally invasive mitral valve repair using loops alone (82.1%) or resection alone (17.9%) with 10-year follow-up (mean, 6.1±4.3 years) (8). Leaflet resection was associated with more ≥2+ mitral regurgitation on predischarge echocardiography (P=0.003) and was a significant predictor of late mortality. Freedom from re-operation was low between both groups at all time points (1, 5, and 10 years) with no statistical significance.
Rationale
Although many reports have focussed on the use of loop neochordae in minimally invasive non-robotic mitral valve repair, there is a paucity of data on its use for robotic surgery (9). The Chord-X loop chordal replacement system (Artivion, Kennesaw, GA, USA) with multiple sutures all attached to the papillary muscle would be cumbersome for robotic surgery. We have simply transposed Mohr’s loop technique from our video-assisted mitral to our robotic practice. Accurate measurement of neochordal length is important and this is more challenging in the totally endoscopic environment. Yahagi et al. have reported success in predicting chord length from the intra-operative transoesophageal echo (TEE), citing the advantage that the measurement can be taken in systole rather than diastole but conceding that this carries a 30% failure rate (10). In our experience, the TEE measurement is important but only a guide, due to the variability of the loop implantation site which is difficult to ascertain exactly with the current resolution of TEE. Refinement of the TEE measurement is always done intra-operatively by the surgeon. Yan’s group have reported use of a short ruler to assess intra-operative chord length during robotic mitral valve repair, but this was not with loop neochordae (11).
There is also a paucity of data about how to vary the effective length of fixed length Gore-Tex loops, particularly lengthening them. No matter how experienced the surgeon, there will always be instances where the selected loop length is suboptimal when the repair is complete. The correct loop length is defined not just by the measured distance from the head of the papillary muscle to the plane of the valve annulus, as identified by a non-prolapsing reference segment, but it also depends on the annuloplasty ring size and the amount of excess tissue in the prolapsing leaflet segment. Okamoto et al. reported use of a loop-in-loop technique to lengthen a short primary set of loops but the authors concede that abrasion between the primary and secondary loops may lead to breakage of the neochordae although that had not occurred in their short-term experience (<1 year) (12).
Objective
In this study, we demonstrate different techniques for accurately measuring loop length in the totally endoscopic environment of robotic surgery. We show how to alter the effective length of fixed length loops, including a novel technique of lengthening loops that eliminates the abrasion between primary and secondary loops of the loop-in-loop technique and therefore obviates the risk of neochordal breakage. We present this article in accordance with the SUPER reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-20/rc).
Pre-operative preparations and requirements
All patients have both transthoracic and TEE echocardiographic imaging prior to surgery, as well as coronary imaging by either computed tomography (CT) or radial angiography. Aorto-ileo-femoral CT scanning allows assessment of access vessel diameter and atherosclerotic burden, planning of port sites, as well as for congenital anomalies, e.g., azygos continuation of the inferior vena cava (IVC).
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this article, the accompanying video, and images.
Step-by-step description
Patient and port positioning
We use the Da Vinci X (Intuitive Surgical, Goleta, CA, USA) with four arms and 8 mm ports, and the thoracoscope in 30°-up orientation. We have TilePro enabled with feeds from the intra-operative TEE and patient vitals. Bluetooth headsets facilitate clear communication between team members (Quail Digital, London, UK) (13). The right chest is elevated approximately 30° using a bolster placed under the right scapula which allows the humeral head to fall posteriorly, allowing unconflicted movement of the left arm port in the third interspace. The access and camera ports should occupy the same interspace, usually the fourth. In our experience, a reliable indicator of this interspace is that which overlies the right inferior pulmonary vein on the chest X-ray. The right arm port goes two interspaces caudal to the access port in the anterior axillary line. The left atrial (LA) retractor port goes in the 4th or 5th interspace, medial to the midclavicular line (14).
Cannulation
We always use internal jugular venous drainage in addition to a femoral venous cannula as this ensures adequate venous drainage in all patients, regardless of body mass. This is of paramount importance as poor venous drainage will compromise right heart protection. Advancing the IVC cannula into the superior vena cava (SVC) allows improved mitral exposure as the cannula acts like a rod in the right atrial (RA), allowing the LA retractor to lift against it. It is therefore advantageous to use a stiffer venous cannula (e.g., Biomedicus, Medtronic, Dublin, Ireland) as opposed to a more flexible one (e.g., LivaNova RAP 23/25-Fr femoral venous, London, UK).
Cardiopulmonary bypass (CPB) and myocardial protection
Due to longer operative times associated with robotic surgery when compared to sternotomy during the learning curve, it is imperative to optimise myocardial protection. A greater degree of systemic cooling (28–30 ℃) than routinely used during a sternotomy (32–34 ℃) protects the right lung from unilateral pulmonary oedema (UPE) (incidence <1%, mortality 33%) (15). Other strategies to prevent UPE include minimising the duration of single lung ventilation, maintenance of mean arterial pressures on CPB ≥65 mmHg, and minimising anaemia.
We use single dose cardioplegia (e.g., Custodiol®) as this avoids the interruption of operative flow associated with redosing of cardioplegia. If using the IntraClude device (Edwards Lifesciences, Irvine, CA, USA), then crystalloid cardioplegia has the advantage of lower line pressures during infusion compared to blood cardioplegia. One pitfall to avoid is repeated saline testing, which washes out the cardioplegia and compromises myocardial protection.
IntraClude or Chitwood transthoracic clamp
The lateral endoscopic approach for robotics (LEAR) technique is a port-based totally endoscopic approach utilising four 8 mm ports and one 20 mm flexible access port (Figure 1) (16). Aortic occlusion is achieved using the IntraClude which needs constant vigilance as its position in the aorta can change leading to innominate artery occlusion. We use an albumin and indocyanine green (ICG) solution to fill the balloon which allows its position to be confirmed by direct vision with the Firefly fluorescence imaging of the Da Vinci system (Video 1) (17). The IntraClude device is introduced via the side arm of the femoral arterial cannula, which reduces the effective luminal area, leading to higher line pressures. It is therefore necessary to place larger, or occasionally bilateral, femoral cannulae to achieve sufficient systemic flow on CPB.
Alternatively, a 4 cm minithoracotomy, similar to that used during a video-assisted ‘mini mitral’ procedure, with an additional three 8 mm ports can be used. This technique was popularised by Dr. Randolph Chitwood (Greenville, NC, USA) who developed the transthoracic aortic clamp (Chitwood clamp). This technique has the advantage that the clamp is reusable and does not migrate during surgery giving more reliable aortic occlusion compared to the IntraClude. Smaller femoral cannulae can be used and bilateral femoral cannulation is rarely required. It does however require a short second CPB run to allow decannulation and control of the aortic root vent site, which can occasionally result in troublesome bleeding. There is also the potential for conflict between the left robotic arm and the clamp.
Measuring loop length in the totally endoscopic environment
There are three techniques which we have illustrated in Video 1:
- Our default technique is to use a pre-knotted CV4 suture through the papillary muscle head which is trimmed both at the level of the non-prolapsing reference point and flush with the papillary muscle. The bedside surgeon then measures this, and loops of that length are implanted, although this can be modified based on the amount of excess tissue in the prolapsing segment;
- A 3 cm paper ruler (these come packaged with the skin marker pens);
- The Mohr chordae gauge (Geister Medizintechnik GmbH, Tuttlingen, Germany), as is widely used for video-assisted mitral surgery.
How to change the effective length of fixed length loops
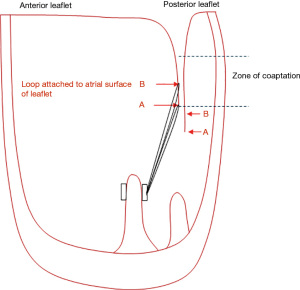
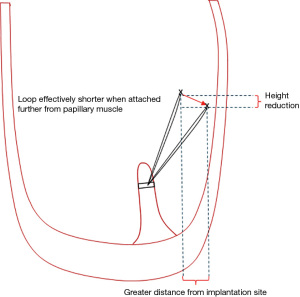
The loop technique facilitates totally endoscopic robotic complex mitral valve reconstruction. Loops save time by avoiding taking multiple bites of the papillary muscle with multiple Gore-Tex sutures. One must remember that the further away from the point of papillary muscle insertion that one attaches the loop, the shorter the loop effectively becomes and this can lead to restriction (Figure 2). Remember that the leaflets are like a sail, they have to be attached at the correct point on the boat (in the heart) to catch the wind (blood). Although one of Mohr’s cardinal rules was not to cross the midline when attaching the loops, the position of the posterior head of the posterior papillary muscle is variable and sometimes lies much closer to or in the midline under a prolapsing P2 than the corresponding posterior head of the anterolateral papillary muscle group. In this circumstance, one may occasionally cross the midline.
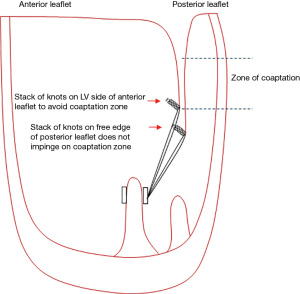
Shortening the loops—simply attach the loop higher up on the atrial surface of the leaflet (Figure 3). If doing this on the anterior leaflet, the knots should be positioned on the ventricular aspect of the leaflet to avoid leaving knots within the zone of coaptation which can lead to small jets of regurgitation (Figure 4).
Lengthening the loops—there are two techniques
(I) Okamoto described a loop-in-loop technique using a reusable neurosurgical aneurysm clip to hold the secondary loop to prevent knot slippage during tying (12). However, the authors acknowledge that there is a risk of abrasion between the primary and secondary loops which may lead to breakage, but that they had not encountered this in the short term (<1 year);
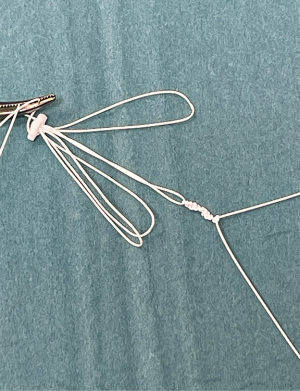
(II) Our preference has always been to simply tie a series of knots to the loop with a second CV4 or CV5 Gore-Tex suture (Figure 5). Each throw adds approximately 0.5 mm to the length of the loop, so a loop would need eight throws to be 4 mm longer. This technique has not been previously described.
Attaching the loops to the leaflets
The basic principle is to avoid placing knots of CV4 or CV5 within the zone of coaptation as this can create small jets of regurgitation (Figure 4). However, the tip of the free edge of the posterior leaflet lies below that of the anterior leaflet once the annuloplasty ring is inserted so the technique of attachment of the loop to the tip of the posterior leaflet is not critical as the knots will lie below the zone of coaptation. The same is not true for the anterior leaflet where the technique of attachment assumes greater importance. Using CV4/5, the leaflet should be picked up first from left ventricle (LV) to LA side, followed by the loop. The suture can then either be tied, or a second bite from LA to LV side can be taken taking care to tie behind the loop (Video 1). This then positions the stack of knots out of the coaptation surface.
Annuloplasty ring
We mark the trigones with ink to ensure accuracy of positioning of the flexible annuloplasty band to the fibrous skeleton of the heart, but also to later aid sizing, and then commence at the left fibrous trigone (LFT) and run anti-clockwise around two-thirds of the mitral annulus. We find sizing is more accurate when most of the annuloplasty sutures are in place as the valve more closely resembles the completed repair. Additionally, the ring size can be modified at this stage based on the depth of coaptation as defined by the ink test in order to mitigate the risk of systolic anterior motion (SAM). We defer placing the last three sutures by the right fibrous trigone (RFT) as these pass in front of the camera. The annuloplasty band is then passed down and starting back at the LFT, the console surgeon sequentially passes the sutures through the band, with the bedside surgeon anchoring the second last suture with CorknotTM (LSI Solutions, Victor, NY, USA) each time. The last three sutures are then placed in sequence once the rest of the band is secured. Alternatively, anchoring the band can start at the RFT and proceed clockwise.
The valve repair is then tested using a motorised saline insufflator (e.g., StrykeFlow, Stryker, Kalamazoo, MI, USA) looking for any regurgitation and the symmetry/position of the closure line. We aim for a symmetrical closure line at least 70% posteriorly and with less than 9–10 mm of A2 coaptation on ink testing—any more than this invariably predicts the risk of SAM. Any imperfections at this stage are easy to correct as the loop is simply detached and either lengthened, shortened, or repositioned. This is one of the most significant advantages of the loop technique compared to individual neochords which cannot be adjusted once tied and have to be explanted.
Atriotomy closure, pacing wire, deairing, and weaning CPB
Use of a pre-knotted CV3 suture to close the left atriotomy saves time tying a set of knots (Figure 6). A LV vent is left across the suture line and then the lungs are inflated to deair the left atrium across the suture line before snugging it down. A pacing wire is placed on the diaphragmatic surface of the right ventricle and exteriorised, and then further deairing manoeuvres are performed by retarding the venous drainage whilst first suctioning the root vent continuously then the LV vent transiently, and the aorta is then unclamped and the heart reperfused. Once cardiac activity has resumed, the venous return is again retarded and suction reapplied to the LV vent and it is removed, and the LA suture line is snugged down again. The competence of the valve repair can now be checked on the TEE and if adequate, the venous retardation is removed, and the LA suture line can be tied down.
The patient is then weaned from CPB, and the repair checked again on a fully loaded heart whilst maintaining suction on the aortic root vent to aid deairing. If deemed adequate and an aortic root cannula has been used, this must be removed and the root secured during a short second CPB run. A 19 Fr Blake drain is placed through the LA retractor port into the oblique sinus and the pericardium is closed with four interrupted 2/0 braided polyester sutures whilst still on CPB. After protamine administration, all the port sites are checked for haemostasis and a 28 Fr straight chest tube placed through the right arm port to lie between the lung and chest wall.
We administer 20 mL liposomal bupivacaine (Exparel, Pacira Biosciences, Tampa, FL, USA) which has been volume expanded with 10 mL of 0.25% levobupivacaine into the right paravertebral gutter and 3rd to 7th intercostal nerves under direct vision using a 21 G butterfly needle and manometer line. We use a butterfly needle as the wings can easily be grasped using a Debakey forceps in the left arm, while lifting the right upper lobe anteriorly off the paravertebral gutter with the right arm.
Postoperative considerations and tasks
We extubate patients in the critical care area, not the operating room, once haemostasis is evident. Chest tubes are removed when serous and <20 mL/h, usually the following morning, prior to transferring to the floor. Post-operative analgesia is with acetaminophen (paracetamol/Tylenol) 1 g per os (po) four times a day (qid) for 5 days. We also use four doses of ketorolac 30 mg intravenous (iv) three times a day (tid) starting on the evening of surgery, followed by ibuprofen 400 mg po tid for a further 5 days starting on post-operative day 2, all covered with lansoprazole 30 mg po od for 4 weeks. We have found the analgesic combination of Exparel with acetaminophen/ketorolac/ibuprofen very effective and reduces opioid consumption. Discharge usually occurs on post-operative day 3 or 4, and return to work 3 weeks later.
Tips and pearls
When moving from a video-assisted ‘mini mitral’ to a robotic platform, minimising the number of new techniques is helpful—transposing the loop technique has been one example of this. We have settled on the pre-knotted CV4 as our default technique for loop length measurement with the length double checked by either the 3 cm paper ruler or Mohr chordae gauge. If the length proves to be suboptimal on the completed repair, simply detach the loops and shorten/lengthen/move them. One advantage over individual neochords, which many tie before implantation of the annuloplasty ring during robotic surgery, is that the loops do not need to be explanted if the length is suboptimal on the completed repair as it can be challenging to obtain adequate access to the subvalvular apparatus when trying to replace individual neochords through a small annuloplasty ring.
Discussion
Strengths and limitations
The loop technique simplifies non-resectional robotic mitral valve repair. It minimizes papillary muscle manipulation, is time efficient and allows broad segments of leaflet prolapse to be corrected with just one papillary muscle anchor point. It confers millimetre accuracy in valve reconstruction and adjustment of neochordal length, which is surprisingly consistent in the human heart. Unlike using individual CV4 neochords which have to be explanted if the length is incorrect after tying, loops can be left in situ and shortened or lengthened to the desired amount. A common misconception of those unfamiliar with this technique is that the length cannot be varied and hopefully we have clarified this. The only limitation compared to individual neochords is that the anchoring knots are tied inside the LV at the papillary muscle level rather than at the leaflet level, which may be more challenging in terms of exposure and technique. However, the greater reach of the robotic arms compared to shafted instruments, and the dynamic LA retractor, are ideal solutions to this.
Implications and actions recommended
The loop technique simplifies the repair of complex valves with multi-segment prolapse and has proven durability during video-assisted ‘mini mitral’ surgery. Its wider adoption for robotic mitral valve repair will confer the same benefits and allow more patients with increasingly complex valves, as well as healthcare systems and wider society, to benefit from totally endoscopic surgery, e.g., short hospital stay and reduced post-operative expenditure, and rapid return to normal activities and work (18-22).
Conclusions
Gore-Tex loops, as pioneered by Mohr, are widely used in Europe for video-assisted ‘mini mitral’ surgery, but their use in robotic surgery has not been widely reported. Their use however is equally applicable to robotic valve repair. Nevertheless, robotics introduces new challenges for the loop technique, such as accurate measurement of loop length in the totally endoscopic environment and hopefully we have now clarified the various techniques available to facilitate this. If the loop length appears suboptimal on the completed repair, the ‘effective length’ can be easily altered without explanting the chords with a series of simple manoeuvres. Loops facilitate complex mitral valve repair of all aetiologies during robotic surgery and are known to give durable and predictable long-term results.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Johannes Bonatti) for the series “Robotic Mitral Valve Repair” published in Journal of Visualized Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-20/rc
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-20/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-20/coif). The series “Robotic Mitral Valve Repair” was commissioned by the editorial office without any funding or sponsorship. PM serves as the unpaid Chairman of the British and Irish Society for Minimally Invasive Cardiac Surgery and an Editorial Board Member of Innovations. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this article, the accompanying video, and images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Frater RW, Vetter HO, Zussa C, et al. Chordal replacement in mitral valve repair. Circulation 1990;82:IV125-30. [PubMed]
- David TE. Replacement of chordae tendineae with expanded polytetrafluoroethylene sutures. J Card Surg 1989;4:286-90. [Crossref] [PubMed]
- David TE, David CM, Lafreniere-Roula M, et al. Long-term outcomes of chordal replacement with expanded polytetrafluoroethylene sutures to repair mitral leaflet prolapse. J Thorac Cardiovasc Surg 2020;160:385-394.e1. [Crossref] [PubMed]
- Mihos CG, Yucel E, Santana O. A systematic review and meta-analysis of chordal replacement versus leaflet resection for isolated posterior mitral valve prolapse. J Cardiovasc Surg (Torino) 2017;58:779-86. [Crossref] [PubMed]
- von Oppell UO, Mohr FW. Chordal replacement for both minimally invasive and conventional mitral valve surgery using premeasured Gore-Tex loops. Ann Thorac Surg 2000;70:2166-8. [Crossref] [PubMed]
- Kuntze T, Borger MA, Falk V, et al. Early and mid-term results of mitral valve repair using premeasured Gore-Tex loops ('loop technique'). Eur J Cardiothorac Surg 2008;33:566-72. [Crossref] [PubMed]
- Falk V, Seeburger J, Czesla M, et al. How does the use of polytetrafluoroethylene neochordae for posterior mitral valve prolapse (loop technique) compare with leaflet resection? A prospective randomized trial. J Thorac Cardiovasc Surg 2008;136:1205-discussion 1205-6. [Crossref] [PubMed]
- Pfannmueller B, Misfeld M, Verevkin A, et al. Loop neochord versus leaflet resection techniques for minimally invasive mitral valve repair: long-term results. Eur J Cardiothorac Surg 2021;59:180-6. [Crossref] [PubMed]
- Fujita T, Kakuta T, Kawamoto N, et al. Benefits of robotically-assisted surgery for complex mitral valve repair. Interact Cardiovasc Thorac Surg 2021;32:417-25. [Crossref] [PubMed]
- Yahagi M, Maeda T, Kanazawa H, et al. Transesophageal echocardiography in robot-assisted mitral valve repair for Barlow's disease: usefulness for predicting artificial ring size and artificial chordae length using the loop technique. JA Clin Rep 2020;6:56. [Crossref] [PubMed]
- Carelli MG, Misfeld M, Wilson-Smith AR, et al. Robotically assisted mitral valve repair-the string, ruler, and bulldog technique. Ann Cardiothorac Surg 2022;11:622-8. [Crossref] [PubMed]
- Okamoto K, Yozu R, Kudo M. Loop-in-loop technique in mitral valve repair via minithoracotomy. Ann Thorac Surg 2012;93:1329-30. [Crossref] [PubMed]
- Tsafrir Z, Janosek-Albright K, Aoun J, et al. The impact of a wireless audio system on communication in robotic-assisted laparoscopic surgery: A prospective controlled trial. PLoS One 2020;15:e0220214. [Crossref] [PubMed]
- Toolan C, Palmer K, Al-Rawi O, et al. Robotic mitral valve surgery: a review and tips for safely negotiating the learning curve. J Thorac Dis 2021;13:1971-81. [Crossref] [PubMed]
- Moss E, Halkos ME, Binongo JN, et al. Prevention of Unilateral Pulmonary Edema Complicating Robotic Mitral Valve Operations. Ann Thorac Surg 2017;103:98-104. [Crossref] [PubMed]
- Murphy DA, Moss E, Binongo J, et al. The Expanding Role of Endoscopic Robotics in Mitral Valve Surgery: 1,257 Consecutive Procedures. Ann Thorac Surg 2015;100:1675-81; discussion 1681-2. [Crossref] [PubMed]
- Yaffee DW, Loulmet DF, Fakiha AG, et al. Fluorescence-guided placement of an endoaortic balloon occlusion device for totally endoscopic robotic mitral valve repair. J Thorac Cardiovasc Surg 2015;149:1456-8. [Crossref] [PubMed]
- Wang A, Brennan JM, Zhang S, et al. Robotic Mitral Valve Repair in Older Individuals: An Analysis of The Society of Thoracic Surgeons Database. Ann Thorac Surg 2018;106:1388-93. [Crossref] [PubMed]
- Coyan G, Wei LM, Althouse A, et al. Robotic mitral valve operations by experienced surgeons are cost-neutral and durable at 1 year. J Thorac Cardiovasc Surg 2018;156:1040-7. [Crossref] [PubMed]
- Paul S, Isaacs AJ, Jalbert J, et al. A population-based analysis of robotic-assisted mitral valve repair. Ann Thorac Surg 2015;99:1546-53. [Crossref] [PubMed]
- Hawkins RB, Mehaffey JH, Mullen MG, et al. A propensity matched analysis of robotic, minimally invasive, and conventional mitral valve surgery. Heart 2018;104:1970-5. [Crossref] [PubMed]
- Mihaljevic T, Jarrett CM, Gillinov AM, et al. Robotic repair of posterior mitral valve prolapse versus conventional approaches: potential realized. J Thorac Cardiovasc Surg 2011;141:72-80.e1-4.
Cite this article as: Massey J, Palmer K, Al-Rawi O, Ridgway T, Modi P. Robotically-assisted mitral valve repair—chordal replacement with Gore-Tex loops. J Vis Surg 2023;9:41.