Uniportal video-assisted thoracoscopic anatomic segmentectomy for small-sized lung cancer
Introduction
With the extensive application of imaging techniques such as high-resolution computed tomography (HRCT) in general and the potential adoption of low-dose spiral CT non-small cell lung cancer (NSCLC) screening for high-risk former smokers, it is likely that a greater number of small lung cancers (<2 cm) and more multiple pure ground-glass opacity (GGO) lesions will be detected (1). Increasing evidence have suggested that video-assisted thoracic surgery (VATS) limited resection especially anatomic segmentectomies are accepted as a valid alternative to lobectomies as it is beneficial in terms of managing the higher potential risks such as the elderly, the breathless and the ones with multiple co-morbidities, better quality of postoperative life, a lower frequency of operative morbidity and increased likelihood of having a second or even a third NSCLC resected, without compromising oncological principles (2). This trend has rapidly changed the clinical practice and new interest in VATS segmentectomies and lymph node assessment has arisen among thoracic surgery communities not only for high-risk but also for good-risk patients with small clinical stage I NSCLC.
Until now, more and more series of uniportal VATS segmentectomy have been published as experience accumulated in VATS lobectomies. Gonzalez-Rivas published the earliest data in uniportal lobectomy and segmentectomy (3). In 2014 Feb I am appreciated to attend the international symposium on uniportal VATS wet-lab and live surgery in Spain by Prof. Diego where I have kept up with the potentially most important trends emerging in thoracic surgery and start our new journey in uniportal approach. In the present paper, we focus on our evolving uniportal experience, the surgical technique and decision-making of uniportal VATS segmentectomy for small-sized lung nodules.
Our uniportal VATS learning curve
I evolve my uniportal approach from thoracoscopy-guided anterior minithoracotomy, then two incisions, not ever using the three or four incisions approach. Tables 1 and 2 summarize our learning curve and a bundle of troubleshooting solutions to the major pitfalls frequently come across in uniportal VATS learning curve. We believe experienced uniportal lobectomy is a prerequisite for starting uniportal segmentectomy (4-6).

Full table

Full table
Uniportal VATS segmentectomy for small-size lung cancer: patient selection and workup
After we have accomplished more than 150 cases of uniportal approach for minor procedures and 30 cases of uniportal lobectomy, uniportal segmentectomy was attempted in 176 patients among which 134 cases of small-size primary lung cancer from Aug 18th 2013 to March 10th 2016 (Table 1).
The indication was either a benign lesion or suspected metastasis in which wedge resection is not possible (42 patients), a suspicion of clinical stage I (≤2 cm in diameter) NSCLC (134 patients). The reason for performing a uniportal segmentectomy for patients with an impaired lung function and/or a previous history of pulmonary resection or multiple pure GGO lesions with concurrent resection or a likelihood of having a second or even a third NSCLC resected in the future. However, in spite of controversy still remaining, most of them are good-risk suspected NSCLC patients with a diameter about 10 mm more or less or less proportion of solid component in our series (7,8). The preliminary data will be discussed in another recently submitted paper. The paper will focus on vital/novel information pertaining to the surgical technique (Table 2).
Several tips are highly recommended based on our experience
Dividing the fissure and segmental hilum using electrocautery (Figure 1)

It has the following potential advantages over stapler:
- Parenchymal theoretically preserving (Figure 2);
- More cost-effective, reduce the number of stapler and cartridges;
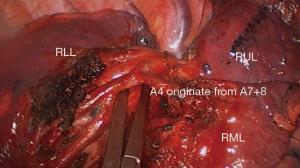
- More precise, prone to identify the possible anomaly and subsegmental vessels (Figure 3);
- To keep the operating field always not bloody and not air leaking is very important to dissect the segmental hilum and expose clearly the deeper vessels.
- Better solution than stapler esp. for complex cases such as calcified lymph node, underdeveloped fissure and so on;
- Without increasing the risk of intraoperative and postoperative air leak and bleeding (Figure 4) (6,10).
Subcarinal lymph node dissection esp. for left side
- For lymph node dissection, suspicion still exists for incomplete resection efficacy in uniportal approach esp. in inexperienced hands;
- Due to esophagus and aorta hindrance, the exposure for left subcarinal lymph node is a more challenging issue;
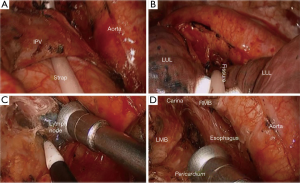
- We resort to dynamic and micro exposure by suction tube (in the left hand of the operator) combining macro exposure by long curved forceps with sponge (in the first assistant hand) (Figure 5) which contribute to the optimal exposure;
- In extremely difficult cases, for example, cardiomegaly, obesity, carina elevation technique by CC Liu’s maneuver (12) to shallow the carina is key to en bloc resection (Figures 6,7).


When uniportal segmentectomy for small-size lung cancer is planned, five main sticking points should be specifically considered
SPN localization
- Because most of these nodules, as you know, are present as more tiny, smaller, softer, deeper, invisible and impalpable, many VATS experts develop or design many different novel localization method (8,14), including but not limited to the following:
- Preoperative CT-guided hook-wire or microcoil or methylene blue staining;
- 3D CTA;
- Thoracoscopy exploration or in vivo finger palpation;
- DynaCT-guided (Hybrid OR);
- Mobile CT (O-arm);
- Intraoperative ultrasonography;
- Radio-guided localization;
- Magnetic navigational bronchoscopy.
- Based on my yet to be identified understanding, almost all the above localization methods share the foundation “the nodule must be found before starting be resected”, that maybe a traditional exploratory thinking in the era of open surgery;
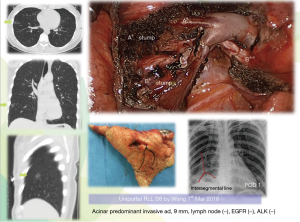
- We believe the small nodule is sure to be palpable after being resected in more than 95% small nodules. We resort to preoperative 3D (coronal, sagittal, cross) CT to verify the definite segment or combined segments, no additional exploratory process, resect the targeted segment, after retrieval, by in vitro finger palpation verify the nodule and sent to frozen section (Figures 4,8);
- However, for wedge resection and subsegmentectomy, also for pure GGO, I strongly recommend localization. Magnetic navigational bronchoscopy guided dye marking is a safe and promising alternative we now apply.

Safe margin
How to make sure safe enough margins? There are different recommendations till today:
- Ginsberg and Rubenstein LCSG: ≥2 cm or margin-to-tumor ratio ≥1;
- Sawabata: > maximum diameter;
- El-Sherif: ≥1 cm.
In fact, Giraud, a radiotherapy expert, tries to quantify the local microscopic extension of small NSCLC. He concluded the extension <8 mm for adenocarcinoma and <6 mm for squamous carcinoma. For segmentectomy in our series, segmental line always 3–5 centimeter beyond which is spacious enough (16,17).
Division of the intersegmental plane (Figure 9)

- Another difficulty faced during uniportal segmentectomy is the identification and division of the intersegmental plane. Several methods have been described;
- The most common is the creation of a ventilated-deflated line by reventilating the operated lung once the segmental bronchus has been stapled. This technique has drawbacks: (I) reventilation obscures the vision and this is a much more troublesome problem than during multiport VATS; (II) the segments to be resected can be partly reventilated through the collateral canals, leading to an unclear demarcation line. Therefore some authors have suggested acting reverse, i.e., reventilating the whole lung once the segmental bronchus has been divided and then collapsing it, so that only the diseased segments remain inflated. We choose the latter;
- For segmental line division, we apply stapler instead of electrocautery often used by Japanese colleagues;
- In uniportal circumstances, stapling is however not that easy because the limited opening of the endostaplers and the thickness of the parenchyma esp. for the segmental line;
- We use long oval forceps shaping the line and guide the stapler introducing the line, then withdrawing the forceps and stapler will easily reach the desired stapling line (6,10) (Figure 9).
Reasonable extent of lymph node dissection in intentional segmentectomy for small-sized lung cancer (Figures 5,7,10)

- From technique point of view, systematic en bloc dissection is safe and feasible (4-6);
- To investigate the metastatic rate of segmental and/or sub-segmental lymph nodes and their roles in pathological staging after a major pulmonary resection, routine examination of level 13/14 lymph nodes is important for accurate pathologic staging and for the predicting clinical outcome of patients with NSCLC;
- However, the reasonable extent of dissection during intentional segmentectomy for peripheral cT1aN0M0 NSCLC are still not highly evidenced;
- Systematic lymph node dissection might not be necessary for ground grass opacity tumors with tumor disappearance rate (TDR) ≥0.25;
- Before bigger data available to guide, the reasonable extent of dissection for intentional segmentectomy for small (≤2 cm) peripheral NSCLC including lymph nodes in the segments with tumors, and the hilar and mediastinal nodes is recommended (20).
When converted to lobectomy in intentional segmentectomy
In experienced pathological center, the precise diagnosis by intraoperative frozen section analysis is an effective method to guide resection strategy for peripheral small-sized lung cancer. The following factors may highly affected the prognosis of ≤2 cm peripheral NSCLC.
- Microscopic positive surgical margin?—absolutely converted to lobectomy or even bigger margin;
- Positive lymph node?—definitely converted to lobectomy;
- Visceral pleural invasion (VPI)?—VPI is a size-independent poor prognostic factor in stage I NSCLC patients. However, VPI may not contribute to the prognosis of patients with part-solid nodules. Thus, upgrading of the TNM stage on the basis of VPI should be carefully considered in these patients (21);
- The existing evidence indicate that adenocarcinoma in situ (AIS) and minimal infiltrating adenocarcinoma (MIA) exhibit good prognosis with 5-year recurrence-free survival rate 100% which may be nominated for limited surgical resection in the future (22);
- However, no sufficiently adequate data concerning IA subgroup analysis and its prognosis exist;
- Infiltrating adenocarcinoma (IA) and its subtype?
- Lepidic predominant IA;
- Acinar- and papillary-predominant IA;
- Solid-type IA;
- Micropapillary predominant (MIP) IA.
- The patients with lepidic-, papillary- and acinar-predominant adenocarcinoma had an intermediate risk group between AIS + MIA and solid-predominant adenocarcinoma. And lepidic-predominant which defined to quantify the non-invasion component (94.9%) is much similar 5 years recurrence-free survival rate with AIS + MIA (100%). For patients with no risk or with ultralow risk of recurrence, excessive surgery may be avoided;
- Solid-type lung cancers demonstrated aggressive behavior, and lymph node metastasis was common in clinical stage IA NSCLC with a solid component. MIP subtype pattern, even at small, early stages, correlates with high lymphovascular invasion, VPI and lymph node metastases. Lobectomy with lymph node dissection remains the standard surgical procedure for patients with solid-type and MIP type, clinical stage IA NSCLC (20);
- The technical challenge now is whether the pathologists are capable of making the subgroup analysis and excluding invasion on the basis of frozen tissue sections and increase concordance rate between frozen section and final pathology. A comprehensive understanding of the biology of invasive tumor size and morphology is vital for therapeutic interventions in the future (23-25).
This paper herein demonstrate several representative segmentectomy whilst reflecting our concerned technical details (Figures 8,11,12).


In conclusion, when uniportal video-assisted thoracoscopic anatomic segmentectomy for small-sized lung cancer is indicated, the decision-making process need to incorporate the potential technical tricks and considering standardization to maximize the benefits of surgery, though more robust prospective study should be performed to validate the efficacy of these predictors.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Cao C, Gupta S, Chandrakumar D, et al. A critical analysis of segmentectomy versus lobectomy for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;46:928-9. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Mendez L, et al. Single-port video-assisted thoracoscopic anatomic segmentectomy and right upper lobectomy. Eur J Cardiothorac Surg 2012;42:e169-71. [Crossref] [PubMed]
- Wang GS, Wang J, Rao ZP, et al. Uniportal complete video-assisted thoracoscopic surgery lobectomy with partial pulmonary arterioplasty for lung cancer with calcified lymph node. J Thorac Dis 2015;7:2366-70. [PubMed]
- Wang GS, Wang Z, Wang J, et al. Uniportal complete video-assisted thoracoscopic lobectomy with systematic lymphadenectomy. J Thorac Dis 2014;6:1011-6. [PubMed]
- Wang GS, Wang Z, Wang J, et al. Biportal complete video-assisted thoracoscopic lobectomy and systematic lymphadenectomy. J Thorac Dis 2013;5:875-81. [PubMed]
- Martin-Ucar AE, Delgado Roel M. Indication for VATS sublobar resections in early lung cancer. J Thorac Dis 2013;5 Suppl 3:S194-9. [PubMed]
- Dai C, Shen J, Ren Y, et al. Choice of Surgical Procedure for Patients With NSCLC ≤ 1 cm or > 1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J Clin Oncol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Wang G, Wang Z, Sun X, et al. Using electrocautery dividing fissure. Asvide 2016;3:360. Available online: http://www.asvide.com/articles/1129
- Ohtsuka T, Goto T, Anraku M, et al. Dissection of lung parenchyma using electrocautery is a safe and acceptable method for anatomical sublobar resection. J Cardiothorac Surg 2012;7:42. [Crossref] [PubMed]
- Wang G, Wang Z, Sun X, et al. Indicating the macro exposure by assistant and micro/dynamic exposure by the operator. Asvide 2016;3:361. Available online: http://www.asvide.com/articles/1130
- Liu CY, Lin CS, Shih CH, et al. Single-port video-assisted thoracoscopic surgery for lung cancer. J Thorac Dis 2014;6:14-21. [PubMed]
- Wang G, Wang Z, Sun X, et al. Using CC Liu’s maneuver for left 7 lymph node dissection. Asvide 2016;3:362. Available online: http://www.asvide.com/articles/1131
- Zaman M, Bilal H, Woo CY, et al. In patients undergoing video-assisted thoracoscopic surgery excision, what is the best way to locate a subcentimetre solitary pulmonary nodule in order to achieve successful excision? Interact Cardiovasc Thorac Surg 2012;15:266-72. [Crossref] [PubMed]
- Wang G, Wang Z, Sun X, et al. Uniportal right lower lobe (RLL) S8 resection. Asvide 2016;3:363. Available online: http://www.asvide.com/articles/1132
- Sienel W, Stremmel C, Kirschbaum A, et al. Frequency of local recurrence following segmentectomy of stage IA non-small cell lung cancer is influenced by segment localisation and width of resection margins--implications for patient selection for segmentectomy. Eur J Cardiothorac Surg 2007;31:522-7; discussion 527-8. [Crossref] [PubMed]
- Giraud P, Antoine M, Larrouy A, et al. Evaluation of microscopic tumor extension in non-small-cell lung cancer for three-dimensional conformal radiotherapy planning. Int J Radiat Oncol Biol Phys 2000;48:1015-24. [Crossref] [PubMed]
- Wang G, Wang Z, Sun X, et al. Division of the intersegmental plane. Asvide 2016;3:364. Available online: http://www.asvide.com/articles/1133
- Wang G, Wang Z, Sun X, et al. Uniportal right mediastinal lymph node dissection. Asvide 2016;3:365. Available online: http://www.asvide.com/articles/1134
- Wang L, Jiang W, Zhan C, et al. Lymph node metastasis in clinical stage IA peripheral lung cancer. Lung Cancer 2015;90:41-6. [Crossref] [PubMed]
- Hattori A, Suzuki K, Matsunaga T, et al. Visceral pleural invasion is not a significant prognostic factor in patients with a part-solid lung cancer. Ann Thorac Surg 2014;98:433-8. [Crossref] [PubMed]
- Liu S, Wang R, Zhang Y, et al. Precise Diagnosis of Intraoperative Frozen Section Is an Effective Method to Guide Resection Strategy for Peripheral Small-Sized Lung Adenocarcinoma. J Clin Oncol 2016;34:307-13. [Crossref] [PubMed]
- Lee MC, Buitrago DH, Kadota K, et al. Recent advances and clinical implications of the micropapillary histological subtype in lung adenocarcinomas. Lung Cancer Manag 2014;3:245-253. [Crossref] [PubMed]
- Yanagawa N, Shiono S, Abiko M, et al. New IASLC/ATS/ERS classification and invasive tumor size are predictive of disease recurrence in stage I lung adenocarcinoma. J Thorac Oncol 2013;8:612-8. [Crossref] [PubMed]
- Jeon HW, Kim YD, Kim KS, et al. Sublobar resection versus lobectomy in solid-type, clinical stage IA, non-small cell lung cancer. World J Surg Oncol 2014;12:215. [Crossref] [PubMed]
- Wang G, Wang Z, Sun X, et al. Uniportal left upper lobe (LUL) trisegmentectomy to show common simple cases. Asvide 2016;3:366. Available online: http://www.asvide.com/articles/1135
- Wang G, Wang Z, Sun X, et al. Uniportal right upper lobe (RUL) S3 resection. Asvide 2016;3:367. Available online: http://www.asvide.com/articles/1136
Cite this article as: Wang G, Wang Z, Sun X, Huang T, Ding G. Uniportal video-assisted thoracoscopic anatomic segmentectomy for small-sized lung cancer. J Vis Surg 2016;2:154.


