Minimally invasive surgical coronary artery revascularization—how to initiate a safe and sustainable program
Introduction
Background
Minimally invasive coronary artery bypass grafting (MI-CABG) approaches that comply with the principles of traditional CABG are rapidly evolving with reported prognostic, quality of life, internal thoracic artery (ITA) graft patency, and patient satisfaction outcomes that are comparable with conventional CABG by sternotomy access (1-8). Recent reports reconfirm the superiority of CABG over percutaneous coronary interventions (PCIs) in various clinical scenarios (9-23) and resulted in renewed interest in minimally invasive surgical revascularization strategies (24-29). Harvesting of the left or bilateral ITA and the subsequent anastomosis to the left anterior descending (LAD) coronary artery and other target vessels under direct vision (30-32) or by using modern endoscopic (33,34) or robotic technology (35-43), with or without the use of cardiopulmonary bypass (CPB), are regarded as the fundamental components of contemporary MI-CABG strategies.
Rationale and knowledge gap
Experienced MI-CABG centres report excellent perioperative, long-term quality of life, and prognostic outcomes (44,45). However, the safe introduction of new MI-CABG programs are potentially deterred by various factors (46-54) that include extensive infrastructure planning, new surgical skill development, implementation of risk management strategies and the mastering of challenging learning curves in an era of decreasing surgical revascularization volume, increased patient expectations, increasing healthcare costs and strict clinical governance (55-60).
Objective
This manuscript provides an overview of contemporary MI-CABG technology and systematically outlines the fundamental aspects of MI-CABG infrastructure design and implementation, current operative approaches and procedural principles, initial patient selection and important risk aversion strategies with the intention of assisting upcoming MI-CABG centres to establish and maintain safe and sustainable programs in both developed and developing countries.
Current minimally invasive surgical coronary revascularization technology
The renewed interest in MI-CABG resulted in exciting advances in less invasive thoracic retractor, three-dimensional (3D) videoscopic/endoscopic, and new generation robotic technology that facilitate direct vision-minimally invasive direct coronary artery bypass (DV-MIDCAB), videoscopic assisted-MIDCAB (VA-MIDCAB), robotic assisted-MIDCAB (RA-MIDCAB), and total endoscopic coronary artery bypass (TECAB) techniques. These technological developments are paralleled by improved target vessel stabilizer, peripheral CPB, and other equipment designs that enable safe and efficient ITA harvesting, excellent coronary artery target vessel access and exposure, operative field stabilization, and anastomotic constructions.
Direct vision retractor, videoscopic, and robotic systems
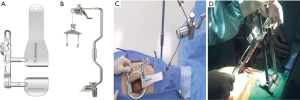
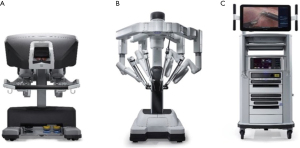
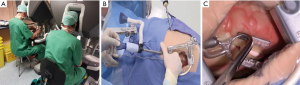
The ThoraTrakTM retractor system (Medtronic, Minneapolis, MN, USA) used in conjunction with the RultractTM Skyhook retractor system (Rultract Inc., Cleveland, OH, USA) and iron assistant (Geister, Tuttlingen, Germany), as well as the TakahasiTM retractor system (Delacroix-Chevalier, Paris, France) are amongst the systems used in DV-MIDCAB to harvest the ITA, perform proximal aorta- and multi-vessel distal coronary artery anastomosis (Figure 1A-1D). The EndoEye Flex HDTM (Olympus, Tokyo, Japan) and the EndoCAMeleonTM videoscopic systems (Karl Storz, Tuttlingen, Germany) are amongst the videoscopic systems available for VA-MIDCAB in combination with special long-shafted endoscopic instruments. The Da VinciTM robotic system (Intuitive, Sunnivale, CA, USA), which consists of a surgical console (Figure 2A) that provide 3D, high-definition videoscopic imaging to manipulate micro-instruments on the patient cart (Figure 2B) through a master controller (Figure 2C) is currently the most widely used robotic system in RA-MIDCAB and TECAB. Other recently introduced robotic systems, each with its own unique design and operational benefits, are progressively being introduced and include the HugoTM (Medtronic, Minneapolis, MN, USA) and VersiusTM (CMR, Cambridge, UK) robotic systems.
Target vessel stabilizers, intra-coronary shunts, and visualization accessories
Target vessel suction stabilizers used in off-pump MI-CABG (Figure 3A,3B) include the Octopus NuvoTM tissue stabilizer (Medtronic, Minneapolis, MN, USA) used in conjunction with the Starfish NSTM heart positioner (Medtronic, Minneapolis, MN, USA), the Genzyme-OPCAB elite system (Genzyme, Boston, MA, USA) and AxiusTM-XposeTM Stabilizer and Apical Positioning Device (Guidant Corporation Cardiac Surgery Group, Indianapolis, IN, USA), while low-profile compressive operative field stabilizers (Figure 3C) include the Terumo Stablesoft II stabilizer (Terumo, Tokyo, Japan). Various intra-coronary shunt designs, which maintain distal perfusion during anastomosis (61) are available and include the Clear ViewTM (Medtronic, Minneapolis, MN, USA), AxiusTM coronary shunt (Guidant Corporation Cardiac Surgery Group, Indianapolis, IN, USA), and NauticaTM (Meril Corporation, Gujurat, India) systems (Figure 3D). Blood-clearing carbon dioxide (CO2) mist-blower devices (Figure 3E) contribute to creating a bloodless operative field and include amongst other the Accumist BlowerTM (Medtronic, Minneapolis, MN, USA).

Peripheral CPB and less invasive cardioplegia delivery technology
The ThruPortTM System (Edwards Lifesciences, Irvine, CA, USA) was originally designed for MI-CABG (62) and consists of an endo-aortic balloon occlusion device that facilitates antegrade cardioplegia delivery, aortic root venting and pressure monitoring, a femoral arterial cannula, femoral venous cannula, a pulmonary catheter vent and a peripheral retrograde cardioplegia catheter. Safe and effective MI-CABG are now commonly performed using peripheral CPB and external aortic clamping with separate antegrade cardioplegia delivery and aortic root venting (63,64).
Automated robotic anastomotic, manual suture knot tying, and conduit flow assessment devices
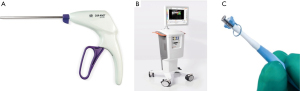
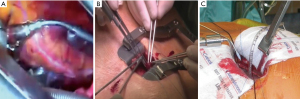
The C-Port Flex-ATM automated anastomotic device (Aesculap, Center Valley, PA, USA) was previously used in TECAB to construct coronary anastomosis with an interrupted row of 13 microscopic stainless steel staples. The extensive cost and lack of clinical and industry support resulted in its unfortunate recent termination, but it remains the only Food and Drug Administration (FDA) approved automated coronary anastomosis device at present (35,39,65,66). The S2 DistalTM Anastomotic System (iiTech Technology Solutions, Pittsburg, PA, USA) and the ELANATM system (AMT Medical, Rosemont, IL, USA) are innovative devices currently under investigation as alternative automated anastomotic technology for TECAB. The Cor-KnotTM mini-suture knotting device (LSI solutions, New York, NY, USA) can be used as an alternative to manual suture knotting (Figure 4A) of MI-CABG anastomosis (67). The value of documenting transit time flow measurements as an indicator of conduit-target vessel patency is well reported (68) and include the MiraQTM system (Medistim, Oslo, Norway), which integrates ultrasound imaging and flow measurement data derived from ultrasound flow probes (Figure 4B,4C).
Infrastructure design and implementation
The introduction of any new surgical procedure requires extensive collaboration between institutional management, quality control, clinical governance, industry, healthcare funder, and clinical teams. The proposal of initiating a state-of-the-art MI-CABG program will be scrutinized and compared against the available clinical outcome data, patient satisfaction, and cost-effectiveness reports for conventional CABG (69-72).
MI-CABG healthcare economics
Contemporary MI-CABG industry role-players provide negotiable rental, leasing, and purchase options for upcoming and established centres. The acquisition and maintenance costs of DV-MIDCAB retractors are substantially less than endoscopic and robotic systems and may be more applicable in developing countries where healthcare cost constraints are challenging. By providing a multi-disciplinary cost-benefit analysis that outlines the return on investment with high-volume use and full occupation of available theatre time, investment in endoscopic or robotic systems may be justified. Arom (69), Leyvi (70), and Pasrija (71) independently reported that MI-CABG procedural costs are offset by reduced post-operative costs, rapid rehabilitation and low readmission rates. Pasrija and colleagues (71) also confirmed that RA-MIDCAB does not increase index hospitalization costs when compared to conventional CABG unless combined with PCI as part of planned hybrid revascularization during the same admission. The significant operational costs of TECAB, which include automated suture devices and other costly consumables, limit its application to expert centres in developed countries.
Hybrid operative theatre design
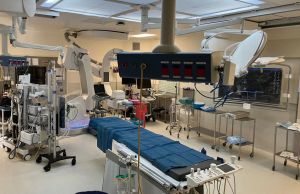
DV-MIDCAB and VA-MIDCAB can be performed in a standard cardiac surgical operating theatre, whereas RA-MIDCAB and TECAB usually require a spacious and dedicated robotic theatre that can facilitate multi-disciplinary use. Modern hybrid cardiovascular operating rooms (Figure 5) are designed to provide an efficient workflow, safe working environment, and unobstructed access to contemporary surgical and transcatheter equipment. Modern MI-CABG operative room design require sufficient ergonomics layouts to accommodate 2 cardiac anaesthetists and ventilator systems, transoesophageal echocardiography (TEE), 2 perfusion technologists and a CPB machine, an endoscopic camera or robotic surgical system, CO2 delivery stack, various unobstructed synchronised monitoring screens, integrated image projection, 2 surgeons, a theatre nurse and a supporting nurse with easy access to all routine cardiovascular equipment, guidewires, grafts, stents, and sutures. Various reports from the United States and Asia suggest a complete return on investment within 2 years of establishing a hybrid theatre (72).
Teamwork, communication, ownership, and leadership
To promote trust and effective teamwork, established centres suggest that a consistent MI-CABG team commit to ongoing training, education, and successful execution of at least the first 20 procedures in close collaboration and partnership with expert centres (45,46,50-52). All team members should be familiar with the theatre and equipment layout, each procedural step, the signs suggestive of ischemia or electrocardiographic changes and how to efficiently manage adverse events. Frequent constructive post-training and postoperative team debriefing sessions that focus on continuous improvement strategies are invaluable and reinforces ownership of each team member under surgical leadership. Effective intraoperative communication is essential and any concerns should be communicated, respected, and immediately addressed. Communication during RA-MIDCAB and TECAB procedures can be enhanced by BluetoothTM headsets and microphones. As the surgeon is isolated from the operative field in RA-MIDCAB and TECAB, potential complication protocols and emergency sternotomy conversions (SC) should be well practised. Skilled intensive care, ward, and outpatient nurses, physiotherapists, other allied health care professionals, the patient’s family and referring physicians form part of an extended post-operative MI-CABG team to ensure continuity of post-operative care (45,50).
Training and education
Industry and expert centres propose a stepwise transition towards MI-CABG by acquiring proven proficiency and competency in multi-vessel complex on-pump CABG by sternotomy access (73), followed by a safe transition to multi-vessel complex off-pump (74) or peripheral CPB assisted CABG, subsequent progression to single vessel MI-CABG and finally multi-vessel peripheral CPB assisted, on-pump, or off-pump MI-CABG with its variations (50,51). Extensive MI-CABG training and education will usually be supervised and supported by industry to ensure that the upcoming team is comfortable, safe, and well-skilled, preferably in partnerships with expert centres (51,52,75). Teams who wish to introduce DV-MIDCAB in their practice need to be well acquainted with the technical aspects and setup of the available retractor systems, whereas VA-MIDCAB programs require training in the use of endoscopic stacks and long shaft instruments. RA-MIDCAB and TECAB programs require extensive training in robotic system setup, docking, draping, instrument exchanges, undocking, console efficiency (Figure 6A) and emergency scenario protocols in compliance with strict industry rules and regulation for accreditation. A combination of online training platforms and high-fidelity drylab, cadaveric wetlab, and animal simulations (76-78) should be utilised for mastering operative field ergonomics (Figure 6B), ITA harvesting, target vessel exposure (Figure 6C), arteriotomy preparation, intra-coronary shunt insertion, a variety of coronary anastomosis techniques (79,80), and training in peripheral CPB techniques (81).
Initial patient selection
Expert centres suggest that high-risk clinical, target vessel, vascular, and echocardiographic characteristics, which are outlined in Table 1, should preferably be excluded during the initial introduction of MI-CABG programs into surgical practices (50). Evaluation of the aorta-iliac-femoral arterial-axis by contrasted computerized tomography (CT), magnetic resonance imaging or an additional peripheral contrast injection during coronary angiography is mandatory if peripheral CPB is considered (50). Safe VA-MIDCAB, RA-MIDCAB, and TECAB instrument manoeuvrability can be predicted by CT-scan measured distances between the chest wall and pericardium being more than 1.7 cm, the distance between the chest wall and LAD being more than 1.5 cm and the antero-posterior to transverse distance thoracic ratio measuring more than 0.45 (45,50).
Table 1
| Patient characteristics |
| Potential difficult access |
| Morbid obesity |
| Thoracic wall deformities |
| Previous left thoracotomy |
| Previous left thoracic radiation or trauma |
| Contraindications to or unsuccessful left lung isolation |
| Previous ilio-femoral peripheral vascular interventions if CPB considered |
| High surgical risk |
| Elderly and high frailty index |
| Previous cardiac surgery |
| Urgent/emergency status: hemodynamic instability, malignant arrhythmia |
| Severe ventricular dysfunction |
| Multi-organ dysfunction |
| Poor respiratory function |
| Vascular disease |
| Aorta-iliac-femoral artery-axis calcification, atheroma, or aneurysms |
| Common femoral artery diameter smaller than 8 mm |
| Ascending aorta ectasia, dilatation, or aneurysm larger than 40 mm |
| Sinu-tubular junction or aortic root dilatation more than 40 mm |
| ITA characteristics |
| Unusable or previously used ITA |
| Left subclavian artery stenosis |
| Coronary artery/target vessel characteristics |
| Diffuse sequential target vessel disease |
| Multiple previous target vessel PCI |
| Intramuscular course |
| Target vessel size ≤1.0 mm |
| Echocardiographic characteristics |
| Poor cardiac function |
| Addition significant valvulopathy |
MI-CABG, minimally invasive coronary artery bypass grafting; CPB, cardiopulmonary bypass; ITA, internal thoracic artery; PCI, percutaneous coronary intervention.
Current MI-CABG approaches, operative principles, and risk aversion strategies
The procedural conduct and technical aspects of DV-MIDCAB, VA-MIDCAB, RA-MIDCAB, and TECAB, with or without the use of peripheral CPB and cardioplegic arrest are well described (30,43,80) and share various common principles.
General patient positioning and setup
Following routine cardiac anaesthesia, MI-CABG patients are positioned supine, with the left hemithorax elevated by an inflatable cushion and the left arm flexed. External defibrillation pads are routinely applied and positioned away from the surgical field, while surgical draping allow immediate full sternotomy and peripheral vascular access (45,50). Single-lung ventilation by double-lumen tube or bronchial blockers is advocated and SC is recommended if lung isolation is inadequate (50). The relevant retractor systems are then assembled in DV-MIDCAB, while the endoscopic or robotic systems used in VA-MIDCAB, RA-MIDCAB, and TECAB are adequately positioned and prepared for installation. If peripheral CPB by femoral access is planned, a right internal jugular venous cannula can be utilized for additional peripheral venous drainage in conjunction with or as an alternative to vacuum assisted drainage (50).
Incisions and port placement
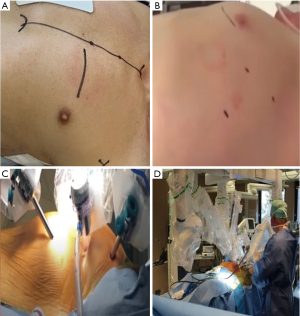
DV-MIDCAB is routinely performed through a left 4–6 cm antero-lateral sub-mammary incision that facilitates intra-thoracic access through the 3rd or 4th intercostal space, after which the relevant retractor blades (Figure 7A) are selected and positioned to allow visualization of the left ITA (30-32). If the ThoraTrakTM, RultractTM Skyhook, and iron assistant systems are used, installation include the placement of the relevant blade into the antero-lateral incision with the tip of the blade extending up to the second rib. The RultractTM can be swivelled superiorly to gain access to the aorta if needed. A 2 cm sub-xyphoid and 6th intercostal anterior axillary incision facilitates the introduction of stabilizer devices once the ITA is harvested. The TakahashiTM MIDCAB retractor system follow the same principles (63,64). VA-MIDCAB (33,34) utilises a 4–6 cm sub-mammary incision as working port in the 4th intercostal space, which is independent from a 1 cm camera port, 5 mm grasper port and 5 mm harmonic scalpel port in the 4th intercostal anterior, 3rd intercostal posterior, and 6th intercostal posterior axillary line, which are used for ITA harvesting (Figure 7B). RA-MIDCAB (35,36,50) and TECAB (37-43) commonly utilise 3 or 4 incisions (Figure 7C) that include 1.0 cm anterior-axillary instrument ports in the 2nd and 6th intercostal spaces and a 1.0-cm camera port in the 4th anterior axillar intercostal space, which will be extended 3–4 cm following ITA harvesting to function as a non-rib-spreading working port in RA-MIDCAB. All ports should be introduced first by blunt dissection after single-lung ventilation is established to determine the presence and extent of lung adhesions, which can carefully be manually dissected from the anterior and lateral chest well to provide sufficient instrument port insertion space and visualization of the ITA. After RA-MIDCAB and TECAB port placement are completed, the surgical table is lowered, rotated 10-degree towards the right and the robotic system subsequently docked (Figure 7D). The surgeon then moves to the console, while a surgical assistant resumes the role of instrument exchanger and other tasks as required. Continuous warm humidified CO2 insufflation at pressures of 8–12 mm of mercury expands the operative field and intra-thoracic workspace without compromising hemodynamic stability and can be decompressed by the insertion of a Veress needle (Endomed Systems, Ravensburg, Germany) through the chest wall.
ITA harvesting, conduit preparation, and proximal aorta anastomosis manoeuvres
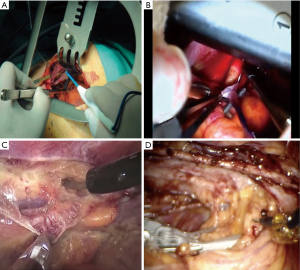
Harvesting of the ITA in MI-CABG follows routine CABG principles. The ITA is carefully dissected from the anterior chest wall in its entire length, either as an in-situ pedicle or as a skeletonized conduit, where the latter is reported to provide additional length, increase flow capacity, easier intrathoracic manoeuvrability, effortless automated-device anastomosis, and more accurate transit-time Doppler flow measurements (68). Low-power diathermy and fine tip instruments are used to avoid side-branch metallic clip contact, excessive traction, grasping, and manipulation. Correct ITA orientation is confirmed before exteriorization and topical and intra-ITA vasodilator therapy are often used as required. The left and right ITA are anatomically close to each other compared to open sternotomy access. In DV-MIDCAB, the left ITA is harvested under direct vision, of which the distal portion is often identified during the initial mini-thoracotomy incision (Figure 8A). As the right ITA is not easily accessible, saphenous vein, or radial artery conduits are commonly used in conjunction with the left ITA and are usually anastomosed to the proximal aorta by various retractor and stabilizer specific manoeuvres that include pericardial traction sutures up to level of the aorta, freeing peri-aortic fat, pushing the pulmonary artery inferiorly and posteriorly and subsequent completion of the proximal anastomosis using a side biting clamp and other routine CABG instruments (Figure 8B). For VA-MIDCAB, long-shafted instruments and diathermy are used to identify the ITA, to dissect the medial and lateral endothoracic facia and to divide ITA branches by combination of diathermy and appropriately sized endothoracic clips (Figure 8C). RA-MIDCAB and TECAB utilize low-power diathermy and robotic clip applicators for full length ITA dissection and branch ligation (Figure 8D). If only a single ITA is harvested, division can be performed after systemic heparinization and haemostasis are ensured. If bilateral ITA conduits are used in VA-MIDCAB, RA-MIDCAB, and TECAB, the right ITA is harvested first, using the same left hemithorax port incisions and ITA harvesting principles as described. A 0-degree robotic endoscope can assist with initial right ITA visualization, dissection of the substernal anterior mediastinal fibro-fatty tissue and right pleural space entrance, after which it is then exchanged with a 30-degree for the remained of the right ITA harvesting (35). The Endo-WristTM stabilizer, which is inserted through a 12 mm subcostal 4th robotic port between the mid-clavicular line and xyphoid process in RA-MIDCAB and TECAB, can compress the anterior mediastinal fat and cardiac border to provide access to the proximal first intercostal branch and distal ITA segment respectively (41). Once the right ITA is harvested, the left ITA is dissected, with neither ITAs divided until coronary target vessels are exposed and prepared. The right ITA is then tunnelled through the anterior mediastinum following systemic heparinization or transsected as a free graft. The videoscopic or robotic systems are subsequently retracted from the operative field in all approaches except for TECAB, after which preparation of the distal ITA is performed through the working port in DV-MIDCAB, VA-MIDCAB, and RA-MIDCAB. Traumatic and atraumatic ITA dysfunction can occur and may warrant SC to ensure satisfactory ITA function and an excellent subsequent prognosis (50).
Target vessel exposure, stabilization, and anastomosis
In DV-MIDCAB, the appropriate retractor blades are installed to allow wide pericardial incision for increased cardiac mobility, target vessel evaluation, stabilizer device insertion or the placement of traction tapes around the left pulmonary vein and inferior vena cava (63,64). The LAD and target vessels coursing on the inferior and lateral walls are easily accessible through various manoeuvres that position the cardiac apex superior-medially. As the proximal venous conduit-aorta anastomosis are usually performed after ITA harvesting, the sequence of revascularization may deviate from traditional beating heart principles by starting at the posterior descending artery, followed by the obtuse marginal targets and eventually the LAD using standard coronary artery surgery instruments (Figure 9A). For off-pump MI-CABG approaches, the coronary arteriotomy is preceded by a team check of hemodynamic and electrocardiographic stability, which is then followed by proximal snare occlusion (Gore Medical, Tempe, AZ, USA), insertion of an appropriately sized intracoronary shunt and release of the snare. The target vessel anastomosis is then constructed with the additional assistance of a blood-clearing CO2 mist device. Inadequate target vessel visualization can potentially be optimised by excluding patients with intramuscular target vessel courses and large epicardial fat distributions as identified by coronary angiography and cardiac CT, careful application of suction- or compression-stabilizing devices and using exposure sutures to manipulate target vessel into the surgical view. By directly visualizing the interventricular septum, the LAD and large diagonal branches can be differentiated. The coronary anastomosis in VA-MIDCAB is similarly performed through a separate antero-lateral incision in the 4th intercostal space (Figure 9B). The camera port in RA-MIDCAB is usually extended into a mini-thoracotomy incision without rib-spreading (Figure 9C), with the sequence of revascularization usually following routine off-pump CABG principles of first targeting the LAD (73,74). For TECAB, an additional 12- or 15-mm sealed port (Ethicon Surgical, Somerville, NJ, USA) is established in the 2nd intercostal space in the midclavicular line, which functions as an access port for coronary shunt insertion, sutures or the automated C-Port Flex ATM device. The pericardial fat pad is dissected free and reflected laterally, after which the pericardium is incised anterior to the phrenic nerve towards the cardiac apex for LAD exposure and posterior to the phrenic nerve for circumflex-marginal target vessel access. Before dividing the ITA and before systemic heparinization, the target vessels are stabilized by the Endo-WristTM stabilizer, carefully exposed and isolated proximally with a SaddleloopTM snare (Quest Medical, Allen, TX, USA). Expert TECAB centres advocate a brief period of monitored ischemic preconditioning (38-44), which allows time to introduce sutures and shunts into the thoracic cavity and to introduce Black DiamondTM forceps into both robotic arms. After the ITA conduits are appropriately tailored to the coronary anatomy, a coronary arteriotomy is performed with an endo-knife (Snap-FitTM; Intuitive Surgical, Sunnyvale, CA, USA), extended as needed, an appropriate sized shunt inserted and the anastomosis constructed by either a continuous running suture or by using an automated C-Port Flex ATM anastomosis device (39,65-66). Ventricle perforation can be avoided by refraining from placing proximal coronary snare sutures through friable myocardium, using soft snares with gentle application and by carefully exposing intramuscular target vessels. Care should be taken to avoid contact or compression of the cardiac structures during robotic instrument manipulation within the right pleural space. Myocardial ischemia in off-pump MI-CABG can be prevented by efficient team communication of electrocardiogram (ECG) changes, ensuring adequate myocardial perfusion pressures, efficient shunt insertion and avoiding suction or compressive device occlusion of distal target vessel outflow. The team at the Catholic University of Leuven emphasised that the hemodynamic instability that potentially occur with cardiac manipulation within the thoracic cavity and the limited access to the aorta for proximal anastomosis, can be minimized by using bilateral ITA conduits in conjunction with other arterial conduits in Y-graft configurations (45).
Procedure conclusion and post-operative care
The pericardium is usually loosely approximated in all MI-CABG approaches after ensuring adequate haemostasis, graft orientation and function. Subsequent wound and incision closures follow routine principles after appropriate sized drains are inserted. Patients are usually extubated 2–6 hours post-operatively, while tailored analgesia, anti-platelet therapy and other medication are administered as needed. Reports that describe the benefits associated with ultra-fast track/in-theatre extubation are progressively emerging with the intention of reducing hospitalization costs and to enhance postoperative recovery (82). Early ambulation and a structured individualised treatment pathway should be supervised by a multi-disciplinary rehabilitation team until home discharge is achieved (Figure 10).
Peripheral CPB and cardioplegic arrest
The anticipation of extensive cardiac manipulation, possible hemodynamic instability and buried target vessels are potential indications for planned peripheral CPB, with or without the use of cardioplegic arrest (35,45,50,62) and is usually performed through a 4-cm right groin incision that provide access to the common femoral vasculature. Total percutaneous cannulation using vascular closure devices can be utilised, but is not advocated if inexperienced or during the initial learning curve (50,81). Peripheral saturation monitoring of the cannulated limb is advised to detect hypoperfusion and the routine use of an additional distal perfusion cannula may be considered (62). External aortic clamping under direct or endoscopic assisted vision (Video 1), antegrade needle cardioplegia delivery and aortic root venting are frequently favoured within the context of cost constraints and availability (63,64). External clamping is preceded by the careful development of the aortic transverse sinus and the introduction of the device through a small separate incision in the 2nd intercostal space. SC is strongly advocated if any difficulties are anticipated or occur (50).
Hybrid coronary artery revascularization
All MI-CABG approaches provide an excellent platform for hybrid revascularization, where the well-established survival benefits of ITA to LAD (83) are combined with contemporary PCI technology that outperform venous conduits for multi-vessel revascularization of non-LAD lesions (18). The benefits of complete revascularization are well reported and randomized control trials that compare conventional CABG with the planned combination of MI-CABG and PCI in different coronary targets within a predefined time-period, suggest similar procedural major adverse cardiac and cerebrovascular events (84-88). PCI is usually performed within 3 to 5 days following MI-CABG (50). Conduit patency and quality can be assessed during the PCI procedure and may potentially offer the additional reported benefits of decreased blood transfusion requirements, shorter hospitalization and rapid regain of preoperative functional status compared to conventional CABG (84-88).
Quality control, clinical governance and need for accreditation
Une (52) reported the safe initiation of MI-CABG without mortality or morbidity by advocating CPB assistance as a risk reduction strategy for SC. The off-pump CABG team at the Catholic University of Leuven (Belgium), reported that the early learning curves do not impact procedural safety and that the need for SC and repeat revascularization decreased significantly after the first 50 procedures (45). The surgical challenges in multi-vessel MI-CABG (89) and risk aversion strategies that aim to improve quality control and clinical governance (50) are well reported. Industry accreditation pathways objectively ensure that appropriate levels of theoretical knowledge and technical skills are mastered, maintained, and further developed.
Conclusions
The ongoing development of MI-CABG techniques and technology create an exciting platform where the traditional benefits of CABG by sternotomy access can be offered to patients with single- or multi-vessel coronary artery disease by less invasive approaches. The application of hybrid coronary revascularization is rapidly expanding and MI-CABG provides the opportunity for improved collaboration between cardiovascular specialities and referral bases. Appropriate infrastructure planning, team training, skills development, low-risk patient selection and collaboration with expert centres form the basis for the successful initiation of a safe and sustainable MI-CABG program. Patients are the greatest advocates of a successful MI-CABG program and every effort to reduce adverse outcome risk within a team context should be the priority.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Visualized Surgery for the series “International Perspectives on Minimally Invasive Coronary Artery Revascularization.” The article has undergone external peer review.
Peer Review File: Available at https://jovs.amegroups.org/article/view/10.21037/jovs-22-43/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.org/article/view/10.21037/jovs-22-43/coif). The series “International Perspectives on Minimally Invasive Coronary Artery Revascularization” was commissioned by the editorial office without any funding or sponsorship. JvdM served as an unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Visualized Surgery from February 2019 to January 2025. FC served as an unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Visualized Surgery from February 2019 to January 2025. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images and video.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Teman NR, Hawkins RB, Charles EJ, et al. Minimally Invasive vs Open Coronary Surgery: A Multi-Institutional Analysis of Cost and Outcomes. Ann Thorac Surg 2021;111:1478-84. [Crossref] [PubMed]
- Liang L, Liu JJ, Kong QY, et al. Comparison of early outcomes associated with coronary artery bypass grafting for multi-vessel disease conducted using minimally invasive or conventional off-pump techniques: a propensity-matched study based on SYNTAX score. J Cardiothorac Surg 2022;17:144. [Crossref] [PubMed]
- Zhang L, Fu Y, Gong Y, et al. Graft patency and completeness of revascularization in minimally invasive multivessel coronary artery bypass surgery. J Card Surg 2021;36:992-7. [Crossref] [PubMed]
- Davierwala PM, Verevkin A, Sgouropoulou S, et al. Minimally invasive coronary bypass surgery with bilateral internal thoracic arteries: Early outcomes and angiographic patency. J Thorac Cardiovasc Surg 2021;162:1109-19.e4. [Crossref] [PubMed]
- Ruel M, Shariff MA, Lapierre H, et al. Results of the Minimally Invasive Coronary Artery Bypass Grafting Angiographic Patency Study. J Thorac Cardiovasc Surg 2014;147:203-8. [Crossref] [PubMed]
- Bonatti J, Ramahi J, Hasan F, et al. Long-term results after robotically assisted coronary bypass surgery. Ann Cardiothorac Surg 2016;5:556-62. [Crossref] [PubMed]
- Halkos ME, Liberman HA, Devireddy C, et al. Early clinical and angiographic outcomes after robotic-assisted coronary artery bypass surgery. J Thorac Cardiovasc Surg 2014;147:179-85. [Crossref] [PubMed]
- Ruel M, Une D, Bonatti J, et al. Minimally invasive coronary artery bypass grafting: is it time for the robot? Curr Opin Cardiol 2013;28:639-45. [Crossref] [PubMed]
- Zhang J, Jiang T, Hou Y, et al. Five-year outcomes comparing percutaneous coronary intervention with drug-eluting stents versus coronary artery bypass grafting in patients with left main coronary artery disease: A systematic review and meta-analysis. Atherosclerosis 2020;308:50-6. [Crossref] [PubMed]
- Wang H, Wang H, Wei Y, et al. Ten-Year Outcomes of Percutaneous Coronary Intervention Versus Coronary Artery Bypass Grafting for Patients with Type 2 Diabetes Mellitus Suffering from Left Main Coronary Disease: A Meta-Analysis. Diabetes Ther 2021;12:1041-54. [Crossref] [PubMed]
- Doenst T, Haverich A, Serruys P, et al. PCI and CABG for Treating Stable Coronary Artery Disease: JACC Review Topic of the Week. J Am Coll Cardiol 2019;73:964-76. [Crossref] [PubMed]
- Lee SE, Lee HY, Cho HJ, et al. Coronary artery bypass graft versus percutaneous coronary intervention in acute heart failure. Heart 2020;106:50-7. [Crossref] [PubMed]
- Farkouh ME, Domanski M, Dangas GD, et al. Long-Term Survival Following Multivessel Revascularization in Patients With Diabetes: The FREEDOM Follow-On Study. J Am Coll Cardiol 2019;73:629-38. [Crossref] [PubMed]
- Ngu JMC, Sun LY, Ruel M. Pivotal contemporary trials of percutaneous coronary intervention vs. coronary artery bypass grafting: a surgical perspective. Ann Cardiothorac Surg 2018;7:527-32. [Crossref] [PubMed]
- Ruel M, Falk V, Farkouh ME, et al. Myocardial Revascularization Trials. Circulation 2018;138:2943-51. [Crossref] [PubMed]
- Farina P, Gaudino MFL, Taggart DP. The Eternal Debate With a Consistent Answer: CABG vs PCI. Semin Thorac Cardiovasc Surg 2020;32:14-20. [Crossref] [PubMed]
- Mulukutla SR, Gleason TG, Sharbaugh M, et al. Coronary Bypass Versus Percutaneous Revascularization in Multivessel Coronary Artery Disease. Ann Thorac Surg 2019;108:474-80. [Crossref] [PubMed]
- Spadaccio C, Benedetto U. Coronary artery bypass grafting (CABG) vs. percutaneous coronary intervention (PCI) in the treatment of multivessel coronary disease: quo vadis? -a review of the evidences on coronary artery disease. Ann Cardiothorac Surg 2018;7:506-15. [Crossref] [PubMed]
- Sipahi I, Akay MH, Dagdelen S, et al. Coronary artery bypass grafting vs percutaneous coronary intervention and long-term mortality and morbidity in multivessel disease: meta-analysis of randomized clinical trials of the arterial grafting and stenting era. JAMA Intern Med 2014;174:223-30. [Crossref] [PubMed]
- Habib RH, Dimitrova KR, Badour SA, et al. CABG Versus PCI: Greater Benefit in Long-Term Outcomes With Multiple Arterial Bypass Grafting. J Am Coll Cardiol 2015;66:1417-27. [Crossref] [PubMed]
- Dangas GD, Farkouh ME, Sleeper LA, et al. Long-term outcome of PCI versus CABG in insulin and non-insulin-treated diabetic patients: results from the FREEDOM trial. J Am Coll Cardiol 2014;64:1189-97. [Crossref] [PubMed]
- Achim A, Leibundgut G. FAME 3 fails to defame coronary artery bypass grafting: what went wrong in the percutaneous coronary intervention arm? Eur J Cardiothorac Surg 2022;62:ezac036. [Crossref] [PubMed]
- Watanabe H, Yamamoto K, Shiomi H, et al. Percutaneous coronary intervention using new-generation drug-eluting stents versus coronary arterial bypass grafting in stable patients with multi-vessel coronary artery disease: From the CREDO-Kyoto PCI/CABG registry Cohort-3. PLoS One 2022;17:e0267906. [Crossref] [PubMed]
- Vallely MP, Hameed I, Gaudino M. Commentary: The evolution of coronary artery bypass surgery: Toward a better operation. J Thorac Cardiovasc Surg 2021;162:1122-4. [Crossref] [PubMed]
- Nambiar P, Kumar S, Mittal CM, et al. Minimally invasive coronary artery bypass grafting with bilateral internal thoracic arteries: Will this be the future? J Thorac Cardiovasc Surg 2018;155:190-7. [Crossref] [PubMed]
- Buehler AM, Ferri C, Flato UA, et al. Robotically assisted coronary artery bypass grafting: a systematic review and meta-analysis. Int J Med Robot 2015;11:150-8. [Crossref] [PubMed]
- Cao C, Indraratna P, Doyle M, et al. A systematic review on robotic coronary artery bypass graft surgery. Ann Cardiothorac Surg 2016;5:530-43. [Crossref] [PubMed]
- Van Praet KM, Kofler M, Shafti TZN, et al. Minimally Invasive Coronary Revascularisation Surgery: A Focused Review of the Available Literature. Interv Cardiol 2021;16:e08. [Crossref] [PubMed]
- Gaudino M, Bakaeen F, Davierwala P, et al. New Strategies for Surgical Myocardial Revascularization. Circulation 2018;138:2160-8. [Crossref] [PubMed]
- Davierwala PM, Verevkin A, Sgouropoulou S, et al. Minimally invasive coronary bypass surgery with bilateral internal thoracic arteries: Early outcomes and angiographic patency. J Thorac Cardiovasc Surg 2021;162:1109-19.e4. [Crossref] [PubMed]
- Boodhwani M, Ruel M, Mesana TG, et al. Minimally invasive direct coronary artery bypass for the treatment of isolated disease of the left anterior descending coronary artery. Can J Surg 2005;48:307-10. [PubMed]
- Holzhey DM, Cornely JP, Rastan AJ, et al. Review of a 13-year single-center experience with minimally invasive direct coronary artery bypass as the primary surgical treatment of coronary artery disease. Heart Surg Forum 2012;15:E61-8. [Crossref] [PubMed]
- Nataf P, Lima L, Regan M, et al. Thoracoscopic internal mammary artery harvesting: technical considerations. Ann Thorac Surg 1997;63:S104-6. [Crossref] [PubMed]
- Ohtsuka T, Wolf RK, Hiratzka LF, et al. Thoracoscopic internal mammary artery harvest for MICABG using the Harmonic Scalpel. Ann Thorac Surg 1997;63:S107-9. [Crossref] [PubMed]
- Amabile A, Komlo C, Van Praet KM, et al. Techniques for Robotic-Assisted Surgical Myocardial Revascularization. Surg Technol Int 2021;39:251-9. [PubMed]
- Amabile A, Torregrossa G, Balkhy HH. Robotic-assisted coronary artery bypass grafting: current knowledge and future perspectives. Minerva Cardioangiol 2020;68:497-510. [Crossref] [PubMed]
- Bonatti J, Wallner S, Crailsheim I, et al. Minimally invasive and robotic coronary artery bypass grafting-a 25-year review. J Thorac Dis 2021;13:1922-44. [Crossref] [PubMed]
- Balkhy HH, Nisivaco S, Kitahara H, et al. Robotic Beating Heart Totally Endoscopic Coronary Artery Bypass in Higher-Risk Patients: Can It be Done Safely? Innovations (Phila) 2018;13:108-13. [Crossref] [PubMed]
- Hashimoto M, Wehman B, Balkhy HH. Robotic totally endoscopic coronary artery bypass: Tips and tricks for using an anastomotic device. J Thorac Cardiovasc Surg 2020;159:e57-60. [Crossref] [PubMed]
- Kitahara H, McCrorey M, Patel B, et al. Benefit of Robotic Beating-Heart Totally Endoscopic Coronary Artery Bypass in Octogenarians. Innovations (Phila) 2019;14:531-6. [Crossref] [PubMed]
- Balkhy HH, Nisivaco S, Kitahara H, et al. Robotic Multivessel Endoscopic Coronary Bypass: Impact of a Beating-Heart Approach With Connectors. Ann Thorac Surg 2019;108:67-73. [Crossref] [PubMed]
- Balkhy HH, Nathan S, Arnsdorf SE, et al. Right Internal Mammary Artery Use in 140 Robotic Totally Endoscopic Coronary Bypass Cases: Toward Multiarterial Grafting. Innovations (Phila) 2017;12:9-14. [Crossref] [PubMed]
- Bonatti J, Vento A, Bonaros N, et al. Robotic totally endoscopic coronary artery bypass grafting (TECAB)-placement of bilateral internal mammary arteries to the left ventricle. Ann Cardiothorac Surg 2016;5:589-92. [Crossref] [PubMed]
- Qureshi SH, Ruel M. The 7 Pillars of Multivessel Minimally Invasive Coronary Surgery. Innovations (Phila) 2021;16:216-7. [Crossref] [PubMed]
- Nambala S, Mishra YK, Ruel M. Less invasive multivessel coronary artery bypass grafting: now is the time. Curr Opin Cardiol 2021;36:735-9. [Crossref] [PubMed]
- Beckmann A, Meyer R, Lewandowski J, et al. German Heart Surgery Report 2019: The Annual Updated Registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac Cardiovasc Surg 2020;68:263-76. [Crossref] [PubMed]
- Whellan DJ, McCarey MM, Taylor BS, et al. Trends in Robotic-Assisted Coronary Artery Bypass Grafts: A Study of The Society of Thoracic Surgeons Adult Cardiac Surgery Database, 2006 to 2012. Ann Thorac Surg 2016;102:140-6. [Crossref] [PubMed]
- Pettinari M, Navarra E, Noirhomme P, et al. The state of robotic cardiac surgery in Europe. Ann Cardiothorac Surg 2017;6:1-8. [Crossref] [PubMed]
- Rodriguez ML, Lapierre HR, Sohmer B, et al. Mid-Term Follow-up of Minimally Invasive Multivessel Coronary Artery Bypass Grafting: Is the Early Learning Phase Detrimental? Innovations (Phila) 2017;12:116-20. [Crossref] [PubMed]
- van der Merwe J, Casselman F, Vermeulen Y, et al. Reasons for Conversion and Adverse Intraoperative Events in Robotically Enhanced Minimally Invasive Coronary Artery Revascularization. Innovations (Phila) 2020;15:251-60. [Crossref] [PubMed]
- Van den Eynde J, Vaesen Bentein H, Decaluwé T, et al. Safe implementation of robotic-assisted minimally invasive direct coronary artery bypass: application of learning curves and cumulative sum analysis. J Thorac Dis 2021;13:4260-70. [Crossref] [PubMed]
- Une D, Lapierre H, Sohmer B, et al. Can minimally invasive coronary artery bypass grafting be initiated and practiced safely?: a learning curve analysis. Innovations (Phila) 2013;8:403-9. [Crossref] [PubMed]
- Christidis NK, Fox SA, Swinamer SA, et al. Reason and Timing for Conversion to Sternotomy in Robotic-Assisted Coronary Artery Bypass Grafting and Patient Outcomes. Innovations (Phila) 2018;13:423-7. [Crossref] [PubMed]
- Trejos AL, Ross I, Scalesse C, et al. Preoperative evaluation of patient anatomy to increase success of robotics-assisted bypass surgery. Innovations (Phila) 2010;5:335-40. [Crossref] [PubMed]
- Shahian DM, Edwards FH, Ferraris VA, et al. Quality measurement in adult cardiac surgery: part 1--Conceptual framework and measure selection. Ann Thorac Surg 2007;83:S3-12. [Crossref] [PubMed]
- O'Brien SM, Shahian DM, DeLong ER, et al. Quality measurement in adult cardiac surgery: part 2--Statistical considerations in composite measure scoring and provider rating. Ann Thorac Surg 2007;83:S13-26. [Crossref] [PubMed]
- Peterson ED, Coombs LP, DeLong ER, et al. Procedural volume as a marker of quality for CABG surgery. JAMA 2004;291:195-201. [Crossref] [PubMed]
- Rathore SS, Epstein AJ, Volpp KG, et al. Hospital coronary artery bypass graft surgery volume and patient mortality, 1998-2000. Ann Surg 2004;239:110-7. [Crossref] [PubMed]
- Shahian DM, Grover FL, Prager RL, et al. The Society of Thoracic Surgeons voluntary public reporting initiative: the first 4 years. Ann Surg 2015;262:526-35; discussion 533-5. [Crossref] [PubMed]
- Campanella P, Vukovic V, Parente P, et al. The impact of Public Reporting on clinical outcomes: a systematic review and meta-analysis. BMC Health Serv Res 2016;16:296. [Crossref] [PubMed]
- Balkhy HH, Quinn CC, Lois KH, et al. Routine intracoronary shunting in multivessel off-pump coronary artery bypass: a retrospective review of in-hospital outcomes in 550 consecutive cases. Heart Surg Forum 2003;6:E32-5. [Crossref] [PubMed]
- Van der Merwe J, Casselman F, Van Praet F. The principles of minimally invasive atrioventricular valve repair surgery utilizing endoaortic balloon occlusion technology: how to start and sustain a safe and effective program. J Vis Surg 2019;5:72. [Crossref]
- Babliak O, Demianenko V, Melnyk Y, et al. Multivessel Arterial Revascularization via Left Anterior Thoracotomy. Semin Thorac Cardiovasc Surg 2020;32:655-62. [Crossref] [PubMed]
- Çaynak B, Sicim H. Routine minimally invasive approach via left anterior mini-thoracotomy in multivessel coronary revascularization. J Card Surg 2022;37:769-76. [Crossref] [PubMed]
- Balkhy HH, Wann LS, Krienbring D, et al. Integrating coronary anastomotic connectors and robotics toward a totally endoscopic beating heart approach: review of 120 cases. Ann Thorac Surg 2011;92:821-7. [Crossref] [PubMed]
- Balkhy HH, Nisivaco SM, Husain AN, et al. The C-Port Distal Coronary Anastomotic Device Is Comparable With a Hand-Sewn Anastomosis: Human Histological Case Study. Innovations (Phila) 2018;13:140-3. [Crossref] [PubMed]
- Jenkin I, Prachee I, Sokal PA, et al. The role of Cor-Knot in the future of cardiac surgery: A systematic review. J Card Surg 2020;35:2987-94. [Crossref] [PubMed]
- Gaudino M, Sandner S, Di Giammarco G, et al. The Use of Intraoperative Transit Time Flow Measurement for Coronary Artery Bypass Surgery: Systematic Review of the Evidence and Expert Opinion Statements. Circulation 2021;144:1160-71. [Crossref] [PubMed]
- Arom KV, Emery RW, Flavin TF, et al. Cost-effectiveness of minimally invasive coronary artery bypass surgery. Ann Thorac Surg 1999;68:1562-6. [Crossref] [PubMed]
- Leyvi G, Schechter CB, Sehgal S, et al. Comparison of Index Hospitalization Costs Between Robotic CABG and Conventional CABG: Implications for Hybrid Coronary Revascularization. J Cardiothorac Vasc Anesth 2016;30:12-8. [Crossref] [PubMed]
- Pasrija C, Kon ZN, Ghoreishi M, et al. Cost and Outcome of Minimally Invasive Techniques for Coronary Surgery Using Robotic Technology. Innovations (Phila) 2018;13:282-6. [Crossref] [PubMed]
- Cronin GM, Schroyer M. Financial aspects of building a hybrid operating suite. In: American Association for Thoracic Surgery 90th Annual Meeting 2010. 2011.
- Buxton BF, Hayward PA. The art of arterial revascularization-total arterial revascularization in patients with triple vessel coronary artery disease. Ann Cardiothorac Surg 2013;2:543-51. [PubMed]
- Puskas JD. Tips and techniques for multivessel OPCAB. Oper Tech Thorac Cardiovasc Surg 2006;11:78-89. [Crossref]
- Yasuda S, Van den Eynde J, Vandendriessche K, et al. Implementation of a beating heart system for training in off-pump and minimally invasive coronary artery bypass. BMC Surg 2021;21:26. [Crossref] [PubMed]
- Reuthebuch O, Lang A, Groscurth P, et al. Advanced training model for beating heart coronary artery surgery: the Zurich heart-trainer. Eur J Cardiothorac Surg 2002;22:244-8. [Crossref] [PubMed]
- Liu X, Yang Y, Meng Q, et al. A Secure and High-Fidelity Live Animal Model for Off-Pump Coronary Bypass Surgery Training. J Surg Educ 2016;73:583-8. [Crossref] [PubMed]
- Ribeiro IB, Ngu JMC, Gill G, et al. Development of a high fidelity pressurized porcine beating heart simulator for cardiac surgery training. Perfusion 2017;32:568-73. [Crossref] [PubMed]
- Lytle BW. Anastomotic techniques. Oper Tech Thorac Cardiovasc Surg 2000;5:222-30. [Crossref]
- Marin-Cuartas M, Sá MP, Torregrossa G, et al. Minimally invasive coronary artery surgery: Robotic and nonrobotic minimally invasive direct coronary artery bypass techniques. JTCVS Tech 2021;10:170-7. [Crossref] [PubMed]
- van der Merwe J, Casselman F, Beelen R, et al. Total Percutaneous Cardiopulmonary Bypass for Robotic and Endoscopic Atrioventricular Valve Surgery. Innovations (Phila) 2017;12:296-9. [Crossref] [PubMed]
- Hayanga HK, Ellison MB, Badhwar V. Patients should be extubated in the operating room after routine cardiac surgery: An inconvenient truth. JTCVS Tech 2021;8:95-9. [Crossref] [PubMed]
- Mohr FW, Davierwala PM. Revascularization strategy for proximal LAD disease: left internal mammary to LAD artery still rules the roost. J Am Coll Cardiol 2014;64:2727-9. [Crossref] [PubMed]
- Blazek S, Rossbach C, Borger MA, et al. Comparison of sirolimus-eluting stenting with minimally invasive bypass surgery for stenosis of the left anterior descending coronary artery: 7-year follow-up of a randomized trial. JACC Cardiovasc Interv 2015;8:30-8. [Crossref] [PubMed]
- Hage A, Giambruno V, Jones P, et al. Hybrid Coronary Revascularization Versus Off-Pump Coronary Artery Bypass Grafting: Comparative Effectiveness Analysis With Long-Term Follow-up. J Am Heart Assoc 2019;8:e014204. [Crossref] [PubMed]
- Giambruno V, Jones P, Khaliel F, et al. Hybrid Coronary Revascularization Versus On-Pump Coronary Artery Bypass Grafting. Ann Thorac Surg 2018;105:1330-5. [Crossref] [PubMed]
- Reynolds AC, King N. Hybrid coronary revascularization versus conventional coronary artery bypass grafting: Systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e11941. [Crossref] [PubMed]
- Ganyukov V, Kochergin N, Shilov A, et al. Randomized Clinical Trial of Surgical vs. Percutaneous vs. Hybrid Revascularization in Multivessel Coronary Artery Disease: Residual Myocardial Ischemia and Clinical Outcomes at One Year-Hybrid coronary REvascularization Versus Stenting or Surgery (HREVS). J Interv Cardiol 2020;2020:5458064. [Crossref] [PubMed]
- Liu JJ, Kong QY, You B, et al. Surgical Challenges in Multi-Vessel Minimally Invasive Coronary Artery Bypass Grafting. J Interv Cardiol 2021;2021:1195613. [Crossref] [PubMed]
Cite this article as: van der Merwe J, Torregrossa G, Dokollari A, Casselman F. Minimally invasive surgical coronary artery revascularization—how to initiate a safe and sustainable program. J Vis Surg 2023;9:36.