Analysis of learning curve for laparoscopic pancreaticoduodenectomy
Introduction
Laparoscopic pancreaticoduodenectomy (LPD) has minimally invasive advantages for selected patients in specialized centers with growing evidence, including smaller surgical incision, reduced intraoperative blood loss, less postoperative pain and earlier recovery compared with open pancreaticoduodenectomy (OPD) (1-4). Similar advantages in the setting of pancreatic ductal adenocarcinoma (PDAC) were also confirmed with a longer progression-free survival in patients undergoing LPD than OPD (5). Moreover, the costs associated with LPD can be balanced when reductions in length of hospital stays (LOS) translated into reduced expenses, which make it comparable with open approach (6).
However, LPD has its inherent challenges and demanding skills as a novel technique, for example, the retroperitoneal location, proximity to major vascular structures, technical difficulty of three anastomoses, and risk of high morbidity and mortality. It was still limited to selected high-volume centers. Just the same as the open approach, a learning curve was supposed to exist for LPD, which may promise new attempts to be routine practice with feasibility and safety once identified and surmounted.
This study aims to analyze the learning curve of the single surgeon by evaluating the changes of clinical outcomes out of the first 120 consecutive LPDs via a prospective staged approach, and share our experience to overcome the learning curve.
Materials and methods
All data of patients were reviewed retrospectively from a prospective database. From September 2012 to July 2015, a series of consecutive LPDs performed by the same surgeon (Dr. Yi-Ping Mou) in the Sir Run Run Shaw Hospital (from September 2012 to June 2015) and the Zhejiang Provincial People’s Hospital (from June 2015 to July 2015) were identified. All the patients had a backup for its perioperative data and recorded video reviewed afterwards. The surgeon had prior experience with OPD and laparoscopic procedure such as radical gastrectomy, distal or central pancreatectomy. All patients were informed of the potential benefits and risks of LPD preoperatively with written consents. And the protocols were conducted following the approval of the Institutional Review Board.
Patients with superior mesenteric vein (SMV)/portal vein (PV) reconstruction and combined multi-visceral resection were excluded. All the data were retrospectively reviewed, including demographic data, operative time (OT), estimated blood loss (EBL), diameter of main pancreatic duct (MPD) and common bile duct (CBD), 90-day morbidity and mortality, LOS, and pathologic results. Postoperative pancreatic fistula (POPF) was defined and graded according to the criteria of International Study Group of Pancreatic Fistula (ISGPF) (7). POPF graded B and C were considered as clinically relevant. Other major postoperative complications were defined and graded by the consensus of International Study Group of Pancreatic Surgery (ISGPS) including delayed gastric emptying (DGE) and post-pancreatectomy hemorrhage (8,9). Bile leakage was defined by the definition of the International Study Group of Liver Surgery (ISGLS) (10).
To analyze the changes in different learning periods of this procedure, the identified 120 cases were divided into four groups according to the surgeon’s staged approach. In the first period (Group A, the first 30 patients), the surgeon focused on the feasibility and safety of LPD. In the second period (Group B, the second 30 patients), the surgeon performed total mesopancreas excision (TMpE) for cancer, aiming to get better oncological outcomes. In the third period (Group C, the third 30 patients), he paid more attention to surgical details, in terms of the sutures. In the fourth period (Group D, the last 30 patients), former primary assistant were trained to perform some steps of LPD, for example, the resection part.
Operative methods
The operative methods used have been described in detail previously. For patients with resectable lesions, we routinely used the modified approach based on “Five Trocars” (11). However, for patients who had SMV encasement with difficulty creating the retro-pancreatic tunnel, that is, borderline resectable pancreatic cancer (BRPC), we used the “Easy First” strategy to perform LPD (12). TMpE had been performed for cancer since Group B. The definition of mesopancreas indicated the soft connective tissue along celiac axis, superior mesenteric vessels and uncinate process of pancreas, especially the lymphatic and nervous structures of retroperitoneal margin as reported (13,14).
After the specimens were removed from the enlarged umbilical port, a frozen section was sent to confirm the negative margins. Then we performed child’s reconstruction in a complete laparoscopic manner following individual construction. Laparoscopic pancreaticojejunostomy (LPJ) was performed using duct-to-mucosa method. If the diameter of MPD was between 2 and 5 mm, LPJ was carried out using interrupted sutures up to 4–6 stitches with stents of proper diameter. As for MPD larger than 5 mm, running sutures were performed without stent. In Group C, we used the non-absorbable sutures instead of absorbable sutures. Laparoscopic choledochojejunostomy (LCJ) was performed with running suture if the CBD was larger than 8 mm, while with interrupted sutures in CBD less than 8 mm. As for the laparoscopic gastrojejunostomy (LGJ), we used an endoscopic linear stapler to perform a side-to-side anastomosis with running sutures to close the common opening.
Statistical analysis
Continuous data was summarized as means ± standard deviations, and the categorized data was expressed as frequencies and percentages. Variants of numeric data with normal distributions in different group were analyzed using analysis of variance (ANOVA) while the non-normally variable were analyzed by Kruskal-Wallis test. And categorical data was tested using Scheffe tests. P value <0.05 was considered statistically significant. For all these analyses, SPSS version 20.0 (Chicago, IL, USA) statistical software was used.
Results
Patient demographics and pathologic outcomes
Between September 2012 and June 2015, 120 patients underwent LPD by single surgeon in our institution. Among them, 111 patients underwent totally LPD, 9 patients underwent laparoscopic assisted pancreaticoduodenectomy (LAPD).
Detailed demographic data was summarized in Table 1. The mean age of patients was 59.7±12.1 (range, 20–85) years old with 80 male and 40 female patients. Average body mass index was 23.4±3.5 (range, 17.4–36.8) kg/m2. A total of 23 (19.2%) patients had a prior surgery. The most common present symptoms at diagnosis were abdominal pain (61, 50.8%) and jaundice (54, 45%). Compared among groups, all of them showed no statistical significance except for the average ages and aged patients older than 80 years old. The average age at diagnosis of Group D is older than the prior three groups (Group D vs. Group A, Group B, Group C; P=0.048, 0.033, 0.006 respectively). There were five aged patients. Of note all of them were in Group D.
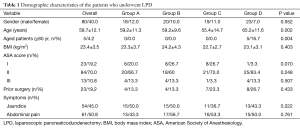
Full table
Pathological results were listed in Table 2, it confirmed malignancy in 85 (70.8%) patients, including PDAC in 36 patients, ampulla of Vater tumor in 32 patients, distal bile duct carcinoma in 14 patients, and other malignancies in 3 patients. Distribution disequilibrium was found only for distal bile duct adenocarcinoma and serous cystic neoplasms. There was no difference of tumor sizes among groups with the cutoff of 5 cm.
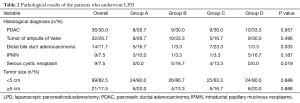
Full table
Operative data and overall clinical outcomes
The operative data and clinical outcomes were shown in Tables 3,4. In general, the mean OT and mean EBL was 359.8±57.6 min, 169.7±152.6 mL respectively. Only 19 (15.8%) patients needed intraoperative blood transfusion. In addition, 42 (35%) patients developed morbidity with no mortality. Clinically relevant pancreatic fistula developed in 12 (10%) cases. Among them there were 9 (7.5%) Grade B POPF, which improved with conservational treatment and 3 (2.5%) Grade C POPF required reoperation. Bile leakage occurred in 4 (3.3%) patients. Twelve patients developed postpancreatectomy hemorrhage, with one managed conservatively, three controlled by embolism with digital subtraction angiography (DSA), eight managed by surgery (including five combined with anastomosis leakage, three Grade C POPF, one bile leakage, one leakage of gastrojejunostomy). Wound infection occurred in 6 (5%) patients, with 3 occurred after reoperation. The average LOS was 17.0±9.8 d.

Full table
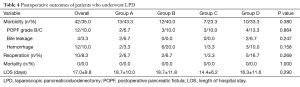
Full table
The mean overall OT tends to decrease continuously from 370.17±52.83 min in Group A to 342±73.10 min in Group D without statistical significance (P=0.092). Resection time of Group A decreased significantly compared with that of Group B and Group C (Group A vs. Group B, Group C; P=0.0062, 0.017 respectively). And it elevated with significance in Group D (Group D vs. Group C; P=0.0088). As for the time of the each anastomosis, mean OT of LPJ had a significant decrease from 55±8.72 min in Group A to 43.57±7.62 min in Group D (P=0.0001), however, it jumped to 55.2±11.9 min (P=0.0017) in Group C with certain reason. The mean time of LCJ decreased continuously from 39.8±11.68 min in Group A to 27.67±11.81 min in Group D (P=0.0036). The diameter of MPD and CBD were comparable (P=0.591, 0.086 respectively). Meanwhile, the EBL decreased from 219.3±147.9 mL in Group A to 140.1±73.6 mL in Group D significantly (P=0.011), at the same time, significant decrease of the transfusion rate was identified (P=0.020). All the postoperative parameters including morbidity, reoperation and LOS remained comparable among groups (P=0.380, 0.269, 0.290 respectively). However, consistent trend of decrease was observed including EBL, morbidity and LOS from 219.33±147.88 mL, 43.3%, 18.67±10.03 d in Group A to 140±73.60 mL, 23.3%, 14.38±6.23 d in Group C respectively with reversed increase in Group D.
Discussion
In this study, we presented our experiences with 120 consecutive LPDs of routine practice by staged approach. Such staged approach was carried out with the expectation of better outcomes or the development of our team. Of note all the LPDs were performed by the same surgeon, and with prospectively established database it may be more objective to analyze the learning curve. To the best of our knowledge, no study analyzed the learning curve by separating OT into resection part and each anastomosis before. The stratification of LPD is essential for individualized treatment, for different operative indications had varied focuses and difficulties over the learning curve. We showed the feasibility and safety of routine practice by surmounting the learning curve with staged approach and the continuous improvements in the clinical outcomes with gained experience.
The overall clinical outcomes of our study were comparable with studies published before. From the view of latitudinal comparison with LPD, Gumbs et al. (2) reviewed the 285 LPDs performed between 1994 and 2010 and conducted a weighted average OT of 371 min with morbidity of 48% and mortality of 2%. The combined POPF and bile leakage rate was 15%. Tran et al. (6) analyzed nationwide outcomes of LPD in United States and concluded a morbidity of 39.4% with an LOS of 11 d. Moreover, hospitals of high volume were associated with decreased complications of 36.7% and reduced LOS of 9 d. Similarly, our experience indicated a mean OT of 359.8±57.6 min with morbidity of 35% and no mortality. Clinically relevant POPF developed in 12 (10%) patients and bile leakage in 4 (3.3%) patients with a mean LOS of 17.0±9.8 d. Increased LOS with comparable morbidity may due to the different discharge criteria based on not only the morbidity but the medical insurance system as well. While compared longitudinally with OPD, Cameron et al. (15) reported 2,000 consecutive OPDs with a morbidity and mortality of 45% and 1.6% respectively, and POPF accounted for 15% of morbidity. Ahmad et al. (16) reported 1,302 patients underwent OPD with a mean OT, intraoperative EBL and overall complications rate of 328.5 min, 626 mL and 44% respectively. Slightly elevated OT with reduced EBL and comparable morbidity were identified. In general, we can conclude that routine practice with staged approach of LPD was feasible and safe.
The learning curve of LPD was identified in several studies published before (1,17-19). Kendrick et al. (1) reported a reduction from a mean time of 7.7 h for the first ten patients to 5.3 h for the latest ten patients. Moreover, Kim et al. (17) reported a decreased OT from 9.8 h for the first 33 patients to 6.6 h for the last 40 patients. And complication rate decreased from 33.3% to 17.6% with shortened LOS from 20.4 to 11.5 d. Song et al. (18) conducted a matched cohort analysis comparing LPD with OPD and concluded that their late LPDs had significantly shorter OTs (399.4 vs. 566.5 min, P<0.001), less EBL (503 vs. 685 mL, P=0.018) and shorter LOS (11.2 vs. 17.3 d, P<0.001) than their early practice. Finally, Speicher et al. (19) also indicated decreased OT and EBL during learning curve, and proposed a staged learning process to surmount learning curve. They believed that learning curve for LPD consisted of a slow and difficult beginning phase, a steep acceleration phase, and finally a plateau phase with slower but continuous improvements.
Similarly, our study confirmed the stead improvements in the clinical outcomes during learning curve as above. Generally, continuous improvements with significant decrease were identified in EBL, transfusion rate and reconstruction time, especially for LPJ and LCJ. But time of LPJ elevated in Group C with significance. Reduced EBL and transfusion may result from the familiarity of “laparoscopic anatomy” and the command of the skills of hemostasis with more precise operation as gained experience, which was of great importance for LPD, especially for TMpE. The laparoscopic reconstruction was critical to perform totally LPD, especially for LPJ and LCJ. Our finding showed clear reduction of time with continuous improvements over the learning curve, especially after the initial 30 patients for LPJ in Group A and initial 60 patients for LCJ. However, this is not to say that LCJ needs a longer learning period than LPJ given the prior experience and insignificant differences in diameter of the ducts. As for the reversed trend of LPJ’s time in Group C, we believed that the switch using from the Vicryl sutures to the Prolene sutures may partly account for that, because it was more difficult to tie the Prolene into knots and it also required more knots (usually 5–6 knots) than Vicryl (Usually 3 knots). We switched Prolene to PDSII in Group D, and a significant reduction was observed.
However, the reduction of reconstruction time was not achieved at the cost of operative quality. No significant difference was found in terms of morbidity (including POPF, bile leakage, hemorrhage), mortality and LOS. Moreover, consistent trend of decrease was observed including EBL, morbidity and LOS from Group A to Group C except for Group D (Figure 1). And the overall OT tended to decrease continuously from 370.17 min (6.2 h) in Group A to 342 min (5.7 h) in Group D. As mentioned above, former primary assistant started to perform several steps of LPD in Group D, such as the resection part, and this can be confirmed by the observed significantly elevated resection time. Moreover, patients in Group D were significantly older than the prior groups, and all aged patients older than 80 years old were in Group D. Reasons above partly explained the slightly reversed trend of clinical outcomes comparable to Group C.

In general, the reconstruction time and clinical outcomes improved mostly after 30 to 60 cases by routine practice, and we concluded that such number was essential to get experienced rather than ten cases as supposed (20), which we believed to represent the difficult beginning phase. As shown, we successfully performed LPDs with staged approach with slower but continuous improvements, just as the plateau phase. The underlying explanation may be that the surgeon followed a prior staged approach before heading for LPD (Figure 2), and gradually extended the indications step by step before routine practice. And he had previous experience on performing laparoscopic radical gastrectomy for lymph node (LN) dissection and LGJ, laparoscopic central pancreatectomy (LCP) for LPJ and laparoscopic operation on congenital biliary cyst for LCJ, which also helped to overcome the initial learning curve. That may explain why Kim et al. (17) and Kendrick et al. (1) reported a steep acceleration phase of rapid learning while we not.
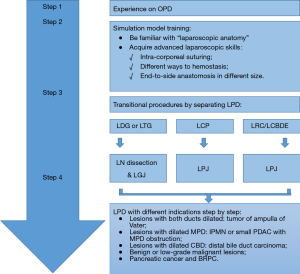
This study has some limitations needed to be pointed out. Firstly, we did not conclude the definite number needed to overcome the learning curve. The main reason lies in the varied indications and the specificity of our previous experience. As mentioned, different operative indications had varied difficulties and focuses in performing LPDs. For example, for ampulla of Vater tumors it was convenient to get the biopsy preoperatively, and the dilation of both ducts makes the LPJ and LCJ much easier. For benign pancreatic head lesion, it’s easier to dissect the lesions with less emphasis on LN dissection, but most patients have smaller MPD and CBD with a fragile pancreatic parenchyma which makes reconstruction challenging. As for pancreatic cancer, it’s technically demanding to resect the lesions en-bloc and get complete lymphadenectomy. And special emphasis on acquiring negative retroperitoneal margin along superior mesenteric vessels was required. Learning curves of this kind can never be assessed only by OT and morbidity, but the oncological outcomes as well. Moreover, given the surgeon’s staged exploration, the learning curve for new adopters may be longer. Secondly, we did not show the oncological outcomes of long-term follow-ups, which needed to be taken into consideration. We plan to analyze and report it in the near future. Last but not least, prospective studies of high quality and comparisons between LPD and OPD are awaited to bring about more objective evaluations.
Conclusions
In conclusion, routine practice of the LPD procedure was feasible and safe in specialized center. Gained experience can improve clinical outcomes in 30 to 60 operations by overcoming the learning curve.
Acknowledgements
Funding: This study was supported by Zhejiang Provincial Key Subject of Medical Science Foundations (Grant No. 11-CX-21) and Zhejiang Provincial Key Project of Research Foundations (Grant No. 2015C03G1360047).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board in the Sir Run Run Shaw Hospital and Zhejiang Provincial People’s Hospital (No. 20160133) and written informed consent was obtained from all patients.
References
- Kendrick ML, Cusati D. Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg 2010;145:19-23. [Crossref] [PubMed]
- Gumbs AA, Rodriguez Rivera AM, et al. Laparoscopic pancreatoduodenectomy: a review of 285 published cases. Ann Surg Oncol 2011;18:1335-41. [Crossref] [PubMed]
- Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg 2012;215:810-9. [Crossref] [PubMed]
- Correa-Gallego C, Dinkelspiel HE, Sulimanoff I, et al. Minimally-invasive vs open pancreaticoduodenectomy: systematic review and meta-analysis. J Am Coll Surg 2014;218:129-39. [Crossref] [PubMed]
- Croome KP, Farnell MB, Que FG, et al. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg 2014;260:633-8; discussion 638-40. [Crossref] [PubMed]
- Tran TB, Dua MM, Worhunsky DJ, et al. The First Decade of Laparoscopic Pancreaticoduodenectomy in the United States: Costs and Outcomes Using the Nationwide Inpatient Sample. Surg Endosc 2016;30:1778-83. [Crossref] [PubMed]
- Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13. [Crossref] [PubMed]
- Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761-8. [Crossref] [PubMed]
- Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142:20-5. [Crossref] [PubMed]
- Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680-8. [Crossref] [PubMed]
- Jin WW, Zhang RC, Mou YP, et al. Modified operative approach for laparoscopic pancreaticoduodenectomy. Chin J Hepat Surg 2014;3:338-40.
- Ren F, Jin WW, Lu C, et al. Clinical efficacy of “Easy First” strategy in laparoscopic pancreaticoduodenectomy for borderline resectable pancreatic cancer. Chin J Dig Surg 2014;14:644-7.
- Gockel I, Domeyer M, Wolloscheck T, et al. Resection of the mesopancreas (RMP): a new surgical classification of a known anatomical space. World J Surg Oncol 2007;5:44. [Crossref] [PubMed]
- Adham M, Singhirunnusorn J. Surgical technique and results of total mesopancreas excision (TMpE) in pancreatic tumors. Eur J Surg Oncol 2012;38:340-5. [Crossref] [PubMed]
- Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg 2015;220:530-6. [Crossref] [PubMed]
- Ahmad SA, Edwards MJ, Sutton JM, et al. Factors influencing readmission after pancreaticoduodenectomy: a multi-institutional study of 1302 patients. Ann Surg 2012;256:529-37. [Crossref] [PubMed]
- Kim SC, Song KB, Jung YS, et al. Short-term clinical outcomes for 100 consecutive cases of laparoscopic pylorus-preserving pancreatoduodenectomy: improvement with surgical experience. Surg Endosc 2013;27:95-103. [Crossref] [PubMed]
- Song KB, Kim SC, Hwang DW, et al. Matched Case-Control Analysis Comparing Laparoscopic and Open Pylorus-preserving Pancreaticoduodenectomy in Patients With Periampullary Tumors. Ann Surg 2015;262:146-55. [Crossref] [PubMed]
- Speicher PJ, Nussbaum DP, White RR, et al. Defining the learning curve for team-based laparoscopic pancreaticoduodenectomy. Ann Surg Oncol 2014;21:4014-9. [Crossref] [PubMed]
- Sharpe SM, Talamonti MS, Wang CE, et al. Early National Experience with Laparoscopic Pancreaticoduodenectomy for Ductal Adenocarcinoma: A Comparison of Laparoscopic Pancreaticoduodenectomy and Open Pancreaticoduodenectomy from the National Cancer Data Base. J Am Coll Surg 2015;221:175-84. [Crossref] [PubMed]
Cite this article as: Lu C, Jin W, Mou YP, Zhou J, Xu X, Xia T, Zhang R, Zhou Y, Yan J, Huang C, Zhang B, Wang J. Analysis of learning curve for laparoscopic pancreaticoduodenectomy. J Vis Surg 2016;2:145.


