Minimally invasive bilateral thoracoscopic maze
Introduction
The minimally invasive approach for atrial fibrillation was developed as an alternative to the technical complexity of the classic maze procedure, as well as a less invasive procedure for treatment of standalone atrial fibrillation. Advances in ablation technology have made minimally invasive approaches feasible, ranging from right mini-thoracotomy to bilateral thoracoscopies. However, the most extensively studied procedure is a bilateral thoracoscopic-mini thoracotomy approach using bipolar radiofrequency ablation. It is described here as part of a staged hybrid approach to the maze procedure developed by our group (1).
Patient selection and workup
Expert consensus considers the indications for surgical treatment of atrial fibrillation to be symptomatic patients with medically refractory AF in whom catheter ablation has failed or who are not candidates for a catheter-based approach (i.e., atrial septal defect devices, anticoagulation intolerance, prior stroke during ablation) or have a low chance of success with a single catheter procedure (large atrium, persistent AF) (2).
Preoperative workup should include a coronary evaluation with a stress test for those patients with no history of angina or coronary artery disease. For those who test positive for ischemia, or for those with a history of angina or coronary artery disease, a cardiac catheterization is performed. Trans-esophageal echocardiogram should be obtained to evaluate for significant valvular disease and left atrial clot. Patients with at least 2+ mitral regurgitation should be considered for concomitant mitral surgery and maze. The presence of left atrial clot is a contraindication to a minimally invasive approach, and such patients should be considered for a trial of therapeutic anticoagulation if they have not already done so. Persistent thrombus should prompt consideration of an open maze. Other relative contraindications include a giant left atrium (>6 cm), pulmonary disease precluding single-lung ventilation, significant coronary artery disease, prior cardiac surgery, and dense pleural adhesions.
Preoperative preparation
Epidural catheter placement is used routinely for postoperative pain control. Initially, the patient is positioned supine. General anesthesia with a double-lumen endotracheal tube is performed followed by TEE to confirm the absence of left atrial clot or significant mitral regurgitation.
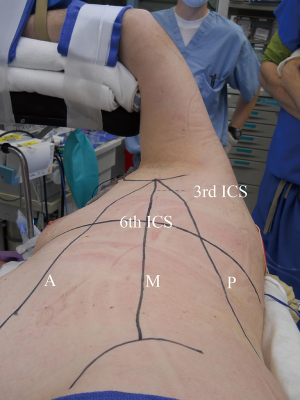
Early in a surgeon’s experience, preparation for the possibility of emergent cardiopulmonary bypass is prudent. While the patient is supine, left femoral arterial and venous lines are placed and prepped into the field during decubitus positioning for access to exchange for cannulas if needed. The patient is placed into the left lateral decubitus position on a beanbag, with a protective axillary roll. The right arm is extended at 90-degrees such that the axilla is exposed. The 3rd and 6th intercostal spaces and the anterior superior iliac spine are marked. The anterior, mid, and posterior axillary lines are marked (Figure 1).

Equipment preference card
- Surgeon headlight;
- 10-mm 30-degree camera;
- 11-mm port;
- Soft-tissue retractor;
- Weitlaner retractors, 2;
- Army Navy retractor;
- Small (10-mm) metal Yankauer suction tube;
- Lumitip Soft Tissue Dissection System (AtriCure);
- Bipolar radiofrequency ablation clamp (Isolator Synergy Clamp, AtriCure);
- Unidirectional radiofrequency ablation device (Isolator Multifunctional Pen, AtriCure);
- Left Atrial Appendage Exclusion System (Atriclip, AtriCure), Endovascular Stapler, or Endoloop;
- Laparoscopic suture passer;
- Endo peanut dissector;
- Endoscopic knot pusher;
- Steri-strips;
- 19-French round Blake drain ×2;
- 28-French straight chest tube.
Procedure
An approximately 5-cm incision is made in the 6th intercostal space at the midaxillary line. An 11-mm port and 30-degree 10-mm camera are inserted. The chest wall is inspected and the 3rd intercostal space identified by a combination of palpation and thoracoscopic visualization (Figure 2). A transverse incision is made in the 3rd intercostal space centered over the midaxillary line (Figure 3).

A soft tissue retractor is inserted. Exposure is improved by use of two Weitlaner retractors (Figures 4,5) and supplemental retraction by the assistant using an army navy retractor.
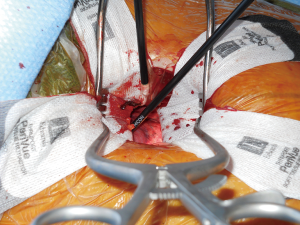
Fat and lymphatics are cleared from the pericardium, and the phrenic nerve is identified. On the right side, this nerve is more easily identified, and nerve injury is best avoided by using sharp technique rather than electrocautery. On the left side, exposure is less straightforward, and bipolar cautery is used as needed for vessels and lymphatics in close proximity to the nerve.
The pericardium is opened sharply 2-cm anterior to the phrenic nerve. Three pericardial stay sutures are placed, brought through the chest wall using a laparoscopic suture passer, and put on retraction using hemostats (Figure 6).

The pericardial reflections are bluntly dissected using an endo peanut dissector. The transverse and oblique sinuses are entered by this dissection. Superiorly, the transverse sinus is dissected beneath the SVC and aorta to create a passageway to the left atrial dome at least halfway across. This dissection also allows one to perform a connecting lesion along the dome of the left atrium. It is possible to also perform the left fibrous trigone lesion to the aortic annulus (i.e., the “Dallas lesion”) (6) although this is not routinely performed by our group.
A 6th intercostal space incision is made at the posterior axillary line. The lighted blunt navigator dissector is inserted through this incision. The tip of the instrument is placed at the superior edge of the right superior pulmonary vein, maintaining the appropriate angle of entry which should be approximately 45-degrees. This indicates the maximum safe depth of insertion of the instrument, and a Steri-strip is used to mark the level at the skin (Figure 7).
The dissector tip is inserted into the oblique sinus and passed posterior to the pulmonary veins, from an inferior-to-superior direction to enter the transverse sinus. To avoid injury, the angle is maintained at 45-degrees and the instrument should be inserted no farther than the marked position. The endo peanut is used to locate the tip and bluntly dissect off any remaining obstructing tissue. The tip is identified by a combination of visualization of the light and tactile sense using the endo peanut. The sleeve is then pulled off the dissector tip and the dissector retracted. The red rubber catheter, which is preattached to the sleeve, is pulled through posterior to the veins. The tip of the catheter is then attached to the bipolar radiofrequency clamp and the clamp then guided around the pulmonary veins (Figure 8).

At least three lines of ablation are delivered, and additional ablation lines applied based on visual inspection of the scar. The multifunctional device is used to identify the major right ganglionated plexi (induction of atrial fibrillation or 50% increase in the R-to-R interval) and ablate them. Conduction entrance and exit block testing are performed. The transverse sinus is further bluntly developed extending midway beneath the SVC to the aortopulmonary window, in order to visualize the left atrial appendage. The unipolar ablation device is used to ablate the left atrial dome (Figure 9).

The pericardium is approximated with 2–0 silk sutures. A right pleural 19-Fr round Blake drain is placed and the right lung is reinflated. The skin incisions are closed in layers. The Blake drain is immediately connected to a drainage system set at −20 cm H2O suction (Figure 10).

The patient is repositioned in the right lateral decubitus position (Figure 11) and ports placed as on the right side.
Dissection and isolation of the left pulmonary veins is performed (Figure 12). The left side dissection is simpler, there being no pericardial reflection as there is on the right side to the IVC. Only the ligament of Marshall need be divided, this being done using bipolar cautery. Conduction block is verified with the testing device. The atrial dome line is completed by creating a line from the base of the appendage to meet the previous ablation line from the right side (Figure 13).


The anatomy and quality of the atrial appendage is assessed and an exclusion device selected. Endoloops are a good choice for narrow appendages. For broad-based appendages an endovascular stapler or clip is more appropriate. We have moved primarily to endoclip devices (Figure 14), due to the risk of bleeding with staplers, particularly when the appendage is fragile and prone to tear. Atraumatic graspers should be used to handle the appendage at all times. A combination of techniques may be used. Once excluded, the appendage is examined for bleeding. A pericardial Blake drain is placed in to the mediastinum and the pericardium closed over it. A left chest tube is inserted. The chest wall sites are inspected for bleeding, and the skin incisions are closed in layers. The patient is repositioned supine for extubation.

Role of team members
Anesthetists should be experienced in epidural catheter placement and double-lumen endotracheal tube positioning. Timely communication regarding changes in oxygenation and hemodynamic status is critical and should prompt immediate discussion for consideration of procedural-related complications.
The assistant should be experienced with use of the 30-degree camera and obtaining adequate views, which are paramount to a safe operation. Since much of the initial work is done through the 3rd intercostal incision under a combination of direct visualization with thoracoscopic assistance, a second assistant dedicated to the camera may be prudent.
Postoperative management
Chest drains are placed on −20 cm H2O suction and changed to bulb suction on the first postoperative day. Removal is considered when 24-hour output is <150 mL. We have found that higher thresholds for removal result in recurrent effusions requiring intervention. Use of non-steroidal anti-inflammatory medications has been also successful in reducing the rate of pleural or pericardial effusions. If renal function is normal, Toradol is administered for 48 hours followed by ibuprofen TID.
Anti-arrhythmic medications are continued postoperatively and anticoagulation (coumadin or direct thrombin inhibitors) are restarted on postoperative day #2. Assessment by electrophysiologists is at 3 and 6 months postoperatively, including monitoring by 48-hr Holter. If the patient remains in sinus rhythm, antiarrhythmics are discontinued at 3 months and anticoagulation at 4 months. Any episode of atrial fibrillation after the 3-month monitoring period should prompt evaluation for trans-septal catheter-based ablation, with lesions connecting the pulmonary vein islands and the mitral annular lesion.
Tips, tricks, and pitfalls
- Adhesions are a relative contraindication to a minimally invasive approach, although if not dense or severe, the described approach can be achieved with a larger incision and a great deal of patience;
- Preoperative transesophageal echocardiogram is mandatory for all cases before skin incision. The thoracoscopic approach should be aborted if TEE demonstrates left atrial thrombus or >2+ MR. Such patients should be re-evaluated for an open approach;
- Inadvertent transdiaphragmatic liver injury during port placement is a risk in morbidly obese patients. Positioning with reverse Trendelenburg and flexion can improve exposure. However, the technique of choice to avoid injury is direct visualization of the thoracic cavity;
- Phrenic nerve injury is best avoided by clear visualization of the course of the nerve. Clearing fat and lymphatics with bipolar cautery and opening the pericardium sharply are techniques to avoid this complication;
- Depending on patient anatomy, the bipolar clamp and camera ports may be exchanged if the angle is more advantageous (See Figure 8, “Right pulmonary vein isolation and ablation”, 0:32 and 1:02). The bipolar clamp may also be inserted from the 3rd intercostal site and passed from a super-to-inferior direction (See Figure 15, “Alternative technique for left pulmonary vein isolation”);
- Continuing the right pleural chest tube to suction is critical during the left-sided procedure, as undrained pleural effusion may affect oxygenation, hemodynamic status, and left-sided visualization;
- Any change in hemodynamic status following exclusion of the left atrial appendage should prompt TEE to assess for changes in lateral wall motion;
- The left atrial appendage should be handled with a padded grasper or endopeanut and with caution to avoid tearing. After application of an exclusion device, it should be inspected for bleeding.

Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lee R, McCarthy PM, Passman RS, et al. Surgical treatment for isolated atrial fibrillation: minimally invasive vs. classic cut and sew maze. Innovations (Phila) 2011;6:373-7. [Crossref] [PubMed]
- Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm 2012;9:632-696.e21. [Crossref] [PubMed]
- Hui DS, Lee R. Positioning. Asvide 2016;3:302. Available online: http://www.asvide.com/articles/1058
- Hui DS, Lee R. Port placements. Asvide 2016;3:303. Available online: http://www.asvide.com/articles/1059
- Hui DS, Lee R. Pericardial incisions and exposure. Asvide 2016;3:304. Available online: http://www.asvide.com/articles/1060
- Edgerton JR, Jackman WM, Mack MJ. A new epicardial lesion set for minimal access left atrial maze: the Dallas lesion set. Ann Thorac Surg 2009;88:1655-7. [Crossref] [PubMed]
- Hui DS, Lee R. Right pulmonary vein dissection and isolation. Asvide 2016;3:305. Available online: http://www.asvide.com/articles/1061
- Hui DS, Lee R. Ablation of right pulmonary veins and ganglionated plexi; entrance/exit block testing. Asvide 2016;3:306. Available online: http://www.asvide.com/articles/1062
- Hui DS, Lee R. Right chest closure. Asvide 2016;3:307. Available online: http://www.asvide.com/articles/1063
- Hui DS, Lee R. Left pulmonary vein dissection and isolation. Asvide 2016;3:308. Available online: http://www.asvide.com/articles/1064
- Hui DS, Lee R. Ablation of left pulmonary veins, ligament of Marshall, ganglionated plexi, and left atrial dome. Asvide 2016;3:309. Available online: http://www.asvide.com/articles/1065
- Hui DS, Lee R. Left atrial appendage exclusion. Asvide 2016;3:310. Available online: http://www.asvide.com/articles/1066
- Hui DS, Lee R. Alternative technique of left pulmonary vein isolation, with catheter exchange for superior-to-inferior passage. Asvide 2016;3:311. Available online: http://www.asvide.com/articles/1067
Cite this article as: Hui DS, Lee R. Minimally invasive bilateral thoracoscopic maze. J Vis Surg 2016;2:133.