Mediastinoscope and laparoscope-assisted esophagectomy
Introduction
Esophageal squamous cell carcinoma (ESCC) shows extensive spread to the mediastinal lymph nodes, especially to those along the bilateral recurrent laryngeal nerves (RLN). Therefore, lymphadenectomy in the upper mediastinum is an essential component of radical esophagectomy for ESCC (1,2). A conventional mediastinoscope has a specialized design created for procedures with a narrow operative field around the tip, and therefore is unsuitable for radical esophagectomy with en bloc lymphadenectomy. In fact, the use of a conventional mediastinoscope has been limited to esophageal mobilization with or without lymph node sampling in mediastinoscope-assisted transhiatal esophagectomy (MATHE) (3-5).
We previously established a transhiatal esophagectomy method with en bloc lymphadenectomy in the middle and lower mediastinum by using a hand-assisted laparoscopic technique (6). Then, we developed a novel cervical procedure for en bloc lymphadenectomy in the upper mediastinum by introducing a single-port laparoscopic technique (7). Our cervical procedure using a single-port ‘mediastinoscope’ can improve the surgical curability and indication of our transhiatal esophagectomy method for esophageal cancer.
Understanding of the mediastinal anatomy specific for cervical and transhiatal approaches is essential for our surgical procedure, and a pneumomediastinum, especially provided from the cervical side, expands the mediastinal space surprisingly, contributing to a safe and careful procedure under video-assisted magnified vision. In this article, we will introduce a novel MATHE procedure with the tips and tricks to perform it safely and carefully.
Patient selection and workup
A resectable thoracic esophageal tumor, evaluated by preoperative CT, was indicated for this operation, irrespective of the presence or absence of any preoperative therapy. Locally advanced tumors with suspicion of invasion to adjacent organs were excluded from this operation, for which conventional open esophagectomy is indicated. Concerning host factors, even in the case of severe pleural adhesion or poor respiratory function, patients who were able to undergo bilateral-lung ventilation were considered as candidates for this operation. Patients with a previous history of gastric cancer surgery were excluded from this operation.
For this operation, preoperative evaluation of the branching pattern of the bilateral bronchial arteries was conducted by 3D-CT angiography.
Equipment preference
The devices used for the single-port cervical technique are shown in Figure 1.

Single-port devices (Hakko, Tokyo, Japan): Lap-protector (FF0707); EZ access port; 5 mm EZ trocar.
Sealing devices: LigaSure Maryland jaw sealer (Shaft length: 44 cm, Covidien, Mansfield, MA, USA) for cervical and transhiatal procedures; EnSeal G2 articulating sealer (Shaft length: 45 cm, Ethicon, Cincinnati, OH, USA) for the transhiatal procedure.
Retractors: Jumbo Hook retractor (Midorijasugiura, Tokyo, Japan) for the cervical procedure; Long retractor (Umihira, Kyoto, Japan) for the transhiatal procedure.
HALS devices: Lap Disc (Ethicon); 12 mm trocar; 5 mm trocar.
Others: 5 mm flexible laparoscope; Endoscopic suction tube with a coagulation electrode (Olympus Medical Systems, Tokyo, Japan); Endoscopic scissors and forceps.
Preoperative preparation
General anesthesia with bilateral lung ventilation was performed using a single lumen endotracheal tube. An epidural anesthesia tube was placed to relieve pain in the upper abdomen. The patient was placed in a supine position with the legs apart. No shoulder roll was placed beneath the shoulders.
Procedure
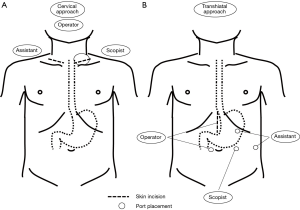
The surgeons’ position, skin incision, and port placement for each approach are shown in Figure 2.
Cervical procedure (Figure 3)

Single-port placement
A left collar incision (4 cm length) was made, and the anterior cervical muscles were divided and the sternocleidomastoid muscle exposed along the inside of the muscle. The cervical esophagus was then mobilized and the cervical lymph nodes along the left RLN were dissected. A lap-protector (Hakko) was inserted into the cervical wound and attached with an EZ access port (Hakko). A pneumomediastinum was created by carbon dioxide insufflation (8 mmHg). Three 5 mm trocars were used to perform the intramediastinal procedure.
Esophageal dissection (left side)
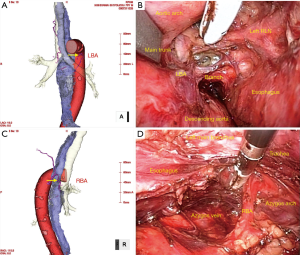
First, the esophagus was dissected along the posterior wall over the aortic arch, as far as LigaSure reached. Then, while avoiding the common carotid, subclavian artery, and aortic arch using a retractor, the left plane of the lymph nodes along the left RLN including the subaortic arch, or tracheobronchial lymph nodes was exposed until the pulmonary arterial wall was reached. The branch of the left bronchial artery (LBA), or sometimes the main trunk of the right bronchial artery (RBA) running across the esophagus anteriorly, was divided.
Esophageal dissection (right side)
Next, moving to the right side of the esophagus, the lymph nodes along the left RLN were separated from the tracheal wall until the tracheobronchial angle was exposed, and the anterior plane of the lymph nodes was exposed until they were separated from the aortic arch. Then, while avoiding the esophagus using a retractor, the esophago-tracheal ligament was divided, and the para-esophageal lymph nodes were dissected along the right mediastinal pleura until exposing the azygos arch, while the RBA was skeletonized. After dividing the esophageal branches of the right vagal nerve, the posterior wall of the esophagus was further dissected along the azygos vein, as far as the device reached. The right plane of the subaortic arch lymph nodes was exposed by separation from the left main bronchus and the pulmonary artery.
Lymphadenectomy along the left RLN
Following esophageal mobilization, the lymph nodes along the left RLN were separated from the left RLN trunk using endoscopic scissors, with the nodes remaining attached to the esophagus. First, the nerve trunk was exposed along the anterior plane, then, the lymph nodes were retracted to the left, through beneath the nerve trunk, and separated by dividing the attachment to the nerve trunk. Finally, the subaortic arch lymph nodes were dissected by dividing the attachment to the nerve trunk.
Lymphadenectomy along the right RLN
The lymph nodes along the right RLN were dissected under direct vision from the right cervical incision. The lateral halves of the anterior cervical muscles were divided to expose the anterior plane of the lymph nodes. Then, the sternocleidomastoid muscle and common carotid artery were retracted to the right to expose the posterior plane of the lymph nodes. Following exposure of the right RLN trunk, the attachment of the lymph nodes to the tracheal wall and the nerve trunk was carefully divided by scissors, then, the lymph nodes were resected.
Transhiatal procedure (Figure 4)

Before the laparoscopic procedure, the greater and lesser omenta were divided under direct vision through the midline incision. Abdominal and transhiatal procedures were performed by carbon dioxide insufflation (10 mmHg). The operator inserted his left hand into the abdominal cavity through a Lap Disc (Ethicon) attached to the midline incision to control the stomach during the abdominal procedure, and to control the esophagus and the liver for hiatal expansion during the transhiatal procedure. After dividing the gastro-splenic ligament, the esophageal hiatus was opened along the left crus, to enter the mediastinum.
Anterior plane dissection
First, following exposure of the pericardium, the anterior plane of the para-esophageal tissues was dissected along the pericardium. After exposing the left inferior pulmonary vein and avoiding it anteriorly with a retractor, the left main bronchial lymph nodes were exposed, separated from the left main bronchus from the periphery to the carina, and further extended to the right to expose the anterior plane of the subcarinal and right main bronchial lymph nodes until the right main bronchus was exposed.
Posterior plane dissection
Next, returning to the hiatus, the aortic wall was exposed, and the posterior plane of the para-esophageal tissues was dissected along the aorta toward the aortic arch, with division of the esophageal branches of the aorta. The posterior plane of the transhiatal dissection was then opened to the space dissected using a cervical approach at the level below the left main bronchus, and the posterior dissection was further extended to the right until the azygos vein and the right mediastinal pleura were exposed.
Left side dissection
Then, the para-esophageal tissues with the para-aortic lymph nodes, dissected using anterior and posterior approaches, were divided along the left mediastinal pleura until the left main bronchus was reached. Consequently, the left mediastinal lymph nodes, including the para-aortic to subcarinal and left main bronchial lymph nodes, were dissected en bloc.
Right side dissection
Then, following complete mobilization of the stomach by dividing the left gastric artery and vein with dissection of the abdominal lymph nodes, the right para-esophageal tissues were divided along the right mediastinal pleura until reaching the right main bronchial lymph nodes, which were then separated from the right main bronchus. Consequently, the right mediastinal lymph nodes, including the right main bronchial lymph nodes, were dissected en bloc.
Esophageal reconstruction
Finally, the cervical esophagus was transected through the left cervical incision, and the esophagus, with the total mediastinal lymph nodes dissected en bloc, was transhiatally removed. A stomach roll was pulled up through a retrosternal route and anastomosed to the esophagus in the left neck.
A J-VAC suction drain (Ethicon) was placed in the left cervical wound. An enteral nutrition tube was inserted through the upper abdominal wall and placed into the jejunum through the stomach roll. Neither a thoracic nor an abdominal drain was placed. No nasogastric tube was placed.
Role of team members
The cervical procedure was performed by the operator in a solo surgery under mediastinoscopic vision provided by the scopist. The operator uses a sealing device in his right hand and a specialized retractor in his left hand during the esophageal dissection, and uses endoscopic scissors and forceps during the sharp dissection along the left RLN. The assistant expands the operative field using retractors in the left cervical procedure before the single-port placement, as well as in the right cervical procedure. During the intramediastinal procedure, the assistant controls the smoke, caused by a sealing procedure, through a trocar. If necessary, the assistant also helps expand the operative field during the sharp dissection around the aortic arch using a retractor through an additional trocar. In contrast, the transhiatal procedure is performed by collaboration between the operator and the assistant. The operator performs the intramediastinal procedure by using a sealing device in his right hand, while controlling the hiatal expansion using his left hand (avoiding the liver, and retracting the esophagus). At the same time, the assistant expands the operative field using the tips of a pair of specialized retractors, while holding the hiatus continuously expanded using the shaft of the retractors.
Postoperative management
The patient was extubated in the operation room, entered the ICU, and then returned to the general ward on postoperative day 1. As this operation does not involve the placement of a thoracic drain, pleural effusion is often detected in either the right or the left pleural cavity by a chest X-ray, and removed by a pleural tap once or twice. The left cervical drain is removed on postoperative day 5. Food intake was started after a swallowing test and vocal cord evaluation by using a laryngoscope after postoperative day 7. The patient was usually discharged from hospital, free from enteral and parenteral nutrition support, 2–3 weeks after the operation.
Tips and tricks
- No placement of a shoulder roll improves accessibility to the anterior plane of the deep mediastinal lymph nodes, such as those around the aortic arch or along the tracheal bifurcation, using cervical and transhiatal approaches;
- Cervical approach followed by a transhiatal approach is essential for en bloc lymphadenectomy around the aortic arch.
Cervical procedure
- The left cervical procedure before the single-port placement is an important step before starting the intramediastinal procedure: dissection should be limited to the minimum requirements to create the air-tight condition, but a small dissection of the mediastinal space along the esophageal wall with a finger is necessary to create the pneumomediastinum, avoiding subcutaneous emphysema. Careful attention should be paid to avoid esophageal or tracheal membranous injury during encircling of the cervical esophagus; taping of the left RLN trunk with its branches is recommended to avoid the risk of RLN palsy;
- During the intramediastinal procedure, the posterior plane dissection along the esophageal wall should be performed first because there are no significant structures to be divided. The deep mediastinal space over the aortic arch can be broadly dissected with pneumomediastinum assistance. This allows the anterior plane and bilateral side dissection along the left RLN to be performed easily and safely;
- The esophagus is mobilized along with the left RLN before the sharp dissection of the lymph nodes along the nerve. Therefore, gently avoiding the esophagus using a retractor is important to prevent RLN palsy during anterior and right side esophageal dissection. You should be careful not to compress the nerve by the retractor. You should be also careful not to use an energy device near the nerve;
- Careful attention should be paid to bronchial artery bleeding and tracheal membranous injury. The bilateral bronchial arteries branch from the descending aorta or the aortic arch, and run across the esophagus anteriorly or posteriorly. The branches or main trunks of the bronchial arteries should be divided for esophageal mobilization or lymphadenectomy around the aortic aorta. Preoperative evaluation of the branching pattern of the bilateral bronchial arteries by 3D-CT angiography is strongly recommended (Figure 5). During the right side esophageal dissection, you should divide the tracheo-esophageal ligament and the para-esophageal lymph nodes individually after separating the lymph nodes from the tracheal wall, to avoid tracheal membranous injury. Divide the ligament along on the esophageal wall.
Transhiatal procedure
- The operator’s left hand is the most effective tool for protecting the stomach as a reconstruction organ during the abdominal procedure, as well for holding the hiatus open and avoiding the liver or stomach during the transhiatal procedure;
- Expansion of the hiatus and the mediastinal space at the same time are important for the transhiatal procedure. Mediastinal dissection is performed with a sealing device in the operator’s right hand, for which the operative field expansion and counter traction using the assistant’s retractors are essential;
- Dissection of the subcarinal and bilateral main bronchial lymph nodes is critical for the transhiatal procedure. Identification of the left main bronchial lymph nodes, while avoiding the left inferior pulmonary vein anteriorly, is the first step in the dissection of these lymph nodes. The left inferior pulmonary vein is easily identified by dissecting cranially on the pericardial surface along the esophageal axis;
- The aortic arch and azygos arch, which are located around the posterior plane of the tracheal bifurcation, are already exposed by the cervical procedure. Therefore, the subcarinal and bilateral main bronchial lymph nodes can be easily dissected by separating the anterior plane from the bilateral main bronchial walls;
- Dissection of the right main bronchial lymph nodes is the most difficult part of the lymphadenectomy along the tracheal bifurcation due to the poor accessibility of a conventional sealing device. ENSEAL G2 Articulating Tissue Sealers significantly improves the handling of the dissection of these lymph nodes;
- During lymphadenectomy along the tracheal bifurcation, the subcarinal dissection often causes bleeding. To avoid bleeding, separate the main bronchial lymph nodes bilaterally from the periphery to the carina, then separate the subcarinal lymph nodes.
Conclusions
The success of video-assisted radical esophagectomy without thoracotomy is determined by whether or not an accurate and sufficient lymphadenectomy in the deep mediastinum is achieved. The elements needed to achieve this include: understanding of the mediastinal anatomy specific for cervical and transhiatal procedures; stable expansion of the mediastinal entry using cervical and transhiatal approaches; adequate expansion of the deep mediastinal space; appropriate use of an energy device; and performing according to the standardized procedure. Based on these elements, improving surgical experience and skills is the most important way to achieve the success of this operation.
Acknowledgements
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by ethics board of Kyoto Prefectural University of Medicine (No. ERB-C-308) and written informed consent was obtained from all patients.
References
- Tachimori Y, Ozawa S, Numasaki H, et al. Efficacy of lymph node dissection by node zones according to tumor location for esophageal squamous cell carcinoma. Esophagus 2016;13:1-7. [Crossref] [PubMed]
- Udagawa H, Ueno M, Shinohara H, et al. The importance of grouping of lymph node stations and rationale of three-field lymphoadenectomy for thoracic esophageal cancer. J Surg Oncol 2012;106:742-7. [Crossref] [PubMed]
- Bumm R, Hölscher AH, Feussner H, et al. Endodissection of the thoracic esophagus. Technique and clinical results in transhiatal esophagectomy. Ann Surg 1993;218:97-104. [Crossref] [PubMed]
- Tangoku A, Yoshino S, Abe T, et al. Mediastinoscope-assisted transhiatal esophagectomy for esophageal cancer. Surg Endosc 2004;18:383-9. [Crossref] [PubMed]
- Feng MX, Wang H, Zhang Y, et al. Minimally invasive esophagectomy for esophageal squamous cell carcinoma: a case-control study of thoracoscope versus mediastinoscope assistance. Surg Endosc 2012;26:1573-8. [Crossref] [PubMed]
- Fujiwara H, Shiozaki A, Konishi H, et al. Hand-assisted laparoscopic transhiatal esophagectomy with a systematic procedure for en bloc infracarinal lymph node dissection. Dis Esophagus 2016;29:131-8. [Crossref] [PubMed]
- Fujiwara H, Shiozaki A, Konishi H, et al. Single-Port Mediastinoscopic Lymphadenectomy Along the Left Recurrent Laryngeal Nerve. Ann Thorac Surg 2015;100:1115-7. [Crossref] [PubMed]
- Fujiwara H, Shiozaki A, Konishi H, et al. The left cervical procedure using a single-port mediastinoscopic technique. Asvide 2016;3:290. Available online: http://www.asvide.com/articles/1052
- Fujiwara H, Shiozaki A, Konishi H, et al. The transhiatal procedure using a hand-assisted laparoscopic technique. Asvide 2016;3:291. Available online: http://www.asvide.com/articles/1053
Cite this article as: Fujiwara H, Shiozaki A, Konishi H, Otsuji E. Mediastinoscope and laparoscope-assisted esophagectomy. J Vis Surg 2016;2:125.


