Transition from thoracotomy to uniportal video-assisted thoracic surgery in non-small cell lung cancer—the Oslo experience
Introduction
As in the rest of the world, lung cancer rates are on the rise in Norway. In women the incidence of lung cancer has increased almost tenfold since the beginning of the 1950’, and in 2014 there were 3,093 new cases of lung cancer in Norway both genders combined. An estimated 20% of all cancer deaths in our country stems from this disease. Besides being the most frequent cause of cancer-related death, lung cancer is the second most prevalent type of cancer in Norwegian men and third most in women, this in a population of just over 5 million people (1).
Norwegian health care services are mainly public, with equal access to treatment for all Norwegian citizens. Furthermore the Cancer Registry of Norway carefully monitors the cancer incidence and their annually published report is an important tool in monitoring cancer diseases. Physicians treating cancer in Norway reports individual cases to the Cancer Registry and the inclusion rate is high.
The resection rate in non-small cell lung cancer (NSCLC) in Norwegian patients has fortunately increases slightly in the last years from aprox. 16% some 10 years ago to aprox. 20% today (2). In the same time period the average TNM stage of patients undergoing surgical treatment have increased. Both of these observations are attributed to a reduction and centralization of centers doing lung resections; at present it is performed in six university clinics and their departments of cardiothoracic surgery. Every patient is discussed at a weekly MDT, which includes oncologists, radiologists, pulmonologists and cardiothoracic surgeons, and an individual plan of treatment is made. 2015 also saw the implementation of a fast-track system; patients with alarm symptoms such as hemoptysis are given a speedy investigation so treatment can start as soon as possible. The work up of every patient follows the European guidelines (3) and recommendations. As to the limitation of surgical treatment, we have for many years performed surgery in single station N2 disease, which we believe to be correct (4). Last year saw a total of 160 NSCLC procedures in our department, and some 800 in Norway as a whole.
Being a small country in terms of population, we have yet to make a formal split in the training of thoracic surgeons. Thus all surgeons performing lung cancer procedures have their boards in cardio-thoracic surgery. This gives the individual surgeon a broad surgical experience, but makes the implementation of new methods, especially advanced procedures, harder. The fact that it has been more than 100 years since the first publication on thoracoscopic surgery by Jacobaeus (5) until we implemented it as our standard approach is testament to this.
History of video-assisted thoracic surgery (VATS) in our institution
From thoracotomy to three-port anterior VATS
As mentioned, we train as cardiothoracic surgeons. The basis of this are boards in general surgery. With increasing laparoscopic procedures being performed in general surgery, trainees in cardiothoracic surgery were questioning why all lung surgery had to be done by thoracotomy. As the number of publications on VATS resections increased and the method gained momentum in the early 2000s, we saw that we needed to implement this method. In 2006 we did our first VATS lobectomy, using the Copenhagen method (6) after a period of visits to Rikshospitalet in Copenhagen, proctoring, courses and following published recommendations (7). Three consultant surgeons trained in the procedure, and we have since reached a VATS rate of aprox 20% of the total number of resections. In our experience, coming from a thoracotomy approach, the three-port approach could feel a little cumbersome. Despite this, we mastered the procedure, but felt that the view and angles of the instruments were a bit “off” especially in relation to the hilar structures.
In 2012 we became aware of the uniportal approach of Dr. Gonzalez-Rivas (8). Initially we did not see the point of reducing the number of ports; our impression was that it would make the surgery more complex with little net result. Thankfully recent years have seen the publication of the possibilities inherent in the procedure, including sleeve resections (9), safe bleeding control (10) and analysis of what sets the method apart from other VATS approaches (11). Fast-forward to the VATS course at IRCAD in Strasbourg where we were able to secure an appointment of a Uniportal Masterclass in our institution with Dr. Gonzalez-Rivas in May 2016.
From three-port anterior to uniportal in a week
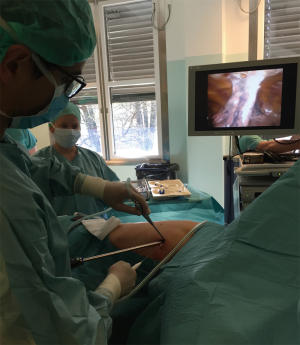
We held the uniportal masterclass in May 2016 at Oslo University Hospital. Together with Dr. Gonzalez-Rivas we did two live cases in addition to the lectures given by him. For the masterclass we chose two cTNM stage I patients with peripheral tumors as the intention was to demonstrate the procedure, and presumptive easier cases were thought to be better for this purpose. Both patients underwent our standard work up in accordance with the European guidelines, including CT guided biopsies, PET-CT, bronchoscopy and lung-function test. Both were discussed at our weekly MDT conference and accepted for surgical treatment. During the master class Dr. Gonzalez-Rivas assisted by Dr. Aamodt performed both procedures (Figure 1).
Case 1
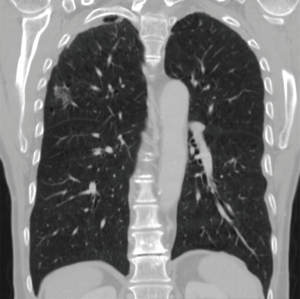
Male, 70 years old. Smoking cessation 6 years ago, previous ENT cancer treated successfully with radiation. Controls for this cancer revealed a tumor in the RUL with a diameter of 23 mm (Figure 2). CT guided biopsy came back as BAC. The patient was in otherwise good clinical condition with acceptable spirometry values with FVC 4.5l (116%), FEV1 3.0l (98%) and DLCO 63%. Bronchoscopy was normal and PET CT was negative for LN disease. Stage given as cT1bN0Mx, stage Ia.
Using general anesthesia and double lumen ventilation, uniportal VATS was performed via the 4th intercostal space using an access port. Adherences to the chest wall were taken down (Figure 3), these were probably stemming from the biopsy procedure. Well-defined fissures apart from the dorsal part. Two apical arteries were prepared; thereafter the bronchus and the upper pulmonary vein were dissected free. The structures were then divided using EndoGia staplers. After dividing the fissure with EndoGia the specimen were removed from the pleural cavity using an EndoCatch 15 cm. Lymph nodes were harvested enbloc from stations # 3, 4R, 7, 9 and 10. Intercostal block in the 3rd–5th ICS using Marcaine 1% 20 mL were applied. Final histology showed an adenocarcinoma with a tumor diameter of 18mm and thus the disease was staged as pT1aN0Mx, stage Ia. The postoperative course was uneventful with the patient requiring only NSAIDs for pain relief and chest drain removal on the 2nd postoperative day.
Case 2
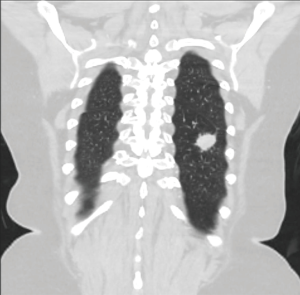
Female, 69 years old, active smoker, preexisting conditions were hypertension, prosthetic hip and obesity (BMI 36). Follow up chest X-ray after orthopedic surgery revealed a tumor in the LLL at 26 mm in diameter, biopsy confirmed SCC (Figure 4). PET CT was negative for LN disease. COPD gold 1–2 with spirometry values of FVC 2.53l (89%), FEV1 1.79 (80%) and DLCO 6.03 (78%). Stage given as cT1bN0Mx, stage Ib.
Using general anesthesia and double lumen ventilation, uniportal VATS was performed via the 4th intercostal space using an access port. The fissures were well defined with a single artery to the LLL clearly visible. After preparation of this, the lover pulmonary vein and the bronchus, the structures were divided using EndoGia staplers and the specimen removed from the pleural cavity using an EndoCatch 15 cm. Lymph nodes were removed enbloc from stations # 5, 7, 9 and 10. Intercostal block in the 3rd–5th ICS were given. Histology of the specimen confirmed the preoperative stage being pT1bN0M0, stage Ia. Uneventful postoperative course, requiring a meager 5 mg iv of ketobemidone in total in addition to paracetamol 1g ×4 and chest drain removal on the 2nd postoperative day.
Learning points from the masterclass
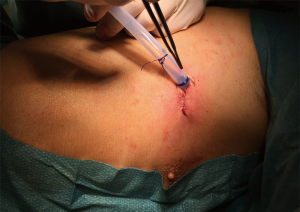
As an institution, we drew four main learning-points from the procedures we saw. Different from the three-port approach, the view during uniportal is much closer to what we are used to during a thoracotomy as it is from above. The instruments are seen below and in an angle to the camera closer to a conventional thoracotomy view than the three-port in our opinion. Secondly, no specialized instruments are needed. Using mainly the suction, an angled clamp and small Foerster clamps, the procedure was fast, safe and controlled. Thirdly, by doing a full dissection of all hilar structures before dividing them we had a safe and straightforward surgery. Last but not least, the postoperative course of both patients was uneventful, the small incisions generating little pain (Figure 5). Drains were out within 48 hrs, and with only an intercostal block in three intercostal spaces, the need for analgesics was meager in both patients, and they were released to their homes within 4 days.
The following week we did two more uniportal lobectomies, and despite longer operating time, the postoperative course in these patients were uneventful as well.
Further perspectives
After the masterclass and our initial positive experience with the uniportal approach, we decided upon making uniportal VATS our institutional standard, allowing for conversion to three ports if deemed necessary perioperatively. The patient logistics for VATS lobectomies have been in place at our institution since 2006, thus the implementation of the uniportal technique demanded little in terms of reorganizing preoperative work up, OR time, postoperative care or strategy of patient mobilization. In terms of surgical equipment we saw that we needed to procure shorter instruments, and upon Dr. Gozalez-Rivas’ recommendation we have purchased sets of his standard tools. In line with this decision, two surgeons are going to the Shanghai Uniportal courses in May 2017. Before that we are planning a proctoring visit by Dr. Gonzalez-Rivas in December 2016.
Tricks and tips
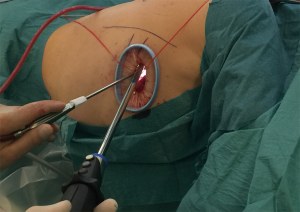
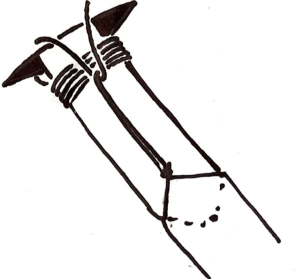
In this early phase of the implementation of the uniportal technique, we more or less follow the recommendations and operative set up shown to us during the masterclass and previously published in several articles (8) and in videos on-line. We have however made one alteration in the operative set up; a vessel loop are being attached to the sterile decking on the hip and shoulder using hemostats (Figure 6) in order to secure the camera in the upper part of the wound and at the same time allowing for easy and quick release of the camera. Furthermore we use our standard “Ullevaal knot” for securing the drain that allows for closure of the wound after removal of the drain (Figure 7).
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Cancer in Norway 2014. Available online: https://www.kreftregisteret.no/Generelt/Publikasjoner/Cancer-in-Norway/Cancer-in-Norway-2014/
- Strand TE, Rostad H, Møller B, et al. Survival after resection for primary lung cancer: a population based study of 3211 resected patients. Thorax 2006;61:710-5. [Crossref] [PubMed]
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010;65 Suppl 3:iii1-27. [Crossref] [PubMed]
- Meacci E, Cesario A, Cusumano G, et al. Surgery for patients with persistent pathological N2 IIIA stage in non-small-cell lung cancer after induction radio-chemotherapy: the microscopic seed of doubt. Eur J Cardiothorac Surg 2011;40:656-63. [PubMed]
- Jacobaeus HC. Uber die möglichkeit die zystoskopie bei Untersuchung seröser höhlungen anzuwenden. Münch Med Wochenschr. 1910;57:2090-2.
- Hansen HJ, Petersen RH, Christensen M. Video-assisted thoracoscopic surgery (VATS) lobectomy using a standardized anterior approach. Surg Endosc 2011;25:1263-9. [Crossref] [PubMed]
- Dunning J, Walker WS. How to set up a VATS lobectomy program. Ann Cardiothorac Surg 2012;1:43-6. [PubMed]
- Gonzalez-Rivas D. Uniportal thoracoscopic surgery: from medical thoracoscopy to non-intubated uniportal video-assisted major pulmonary resections. Ann Cardiothorac Surg 2016;5:85-91. [Crossref] [PubMed]
- Gonzalez-Rivas D, Yang Y, Stupnik T, et al. Uniportal video-assisted thoracoscopic bronchovascular, tracheal and carinal sleeve resections†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i6-16. [PubMed]
- Gonzalez-Rivas D, Stupnik T, Fernandez R, et al. Intraoperative bleeding control by uniportal video-assisted thoracoscopic surgery†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i17-24. [PubMed]
- Bertolaccini L, Viti A, Terzi A, et al. Geometric and ergonomic characteristics of the uniportal video-assisted thoracoscopic surgery (VATS) approach. Ann Cardiothorac Surg 2016;5:118-22. [Crossref] [PubMed]
Cite this article as: Aamodt H. Transition from thoracotomy to uniportal video-assisted thoracic surgery in non-small cell lung cancer—the Oslo experience. J Vis Surg 2016;2:111.